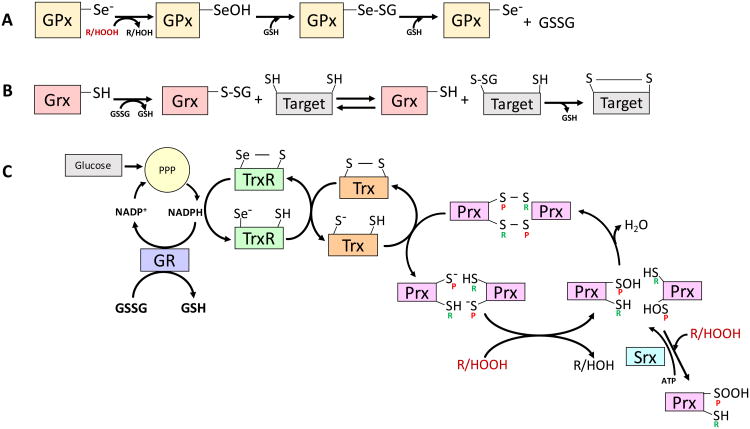
Figure 3.
The Defenders and Their Reactions. A, The catalytic site of glutathione peroxidases (GPx) uses selenocysteine. Relative to sulfur, the reduced pKa of selenium (Se) makes it more readily ionizable at physiological pH. In the GPx peroxidation reaction the selenite anion (Se-) is oxidized by a peroxide to the selenic acid form (-SeOH), reducing the peroxide to a hydroxide in the process. The selenic acid group in GPx-SeOH can be glutathionylated, giving the mixed selenide-sulfide Se-SG, which can be attacked by a second glutathione (GSH) molecule to generate oxidized glutathione (GSSG) and restore the enzyme to its starting reduced form. B, Glutaredoxin (Grx) mediates reversible transfer reactions of glutathione involving glutathionylated protein targets. C, Peroxiredoxins (Prx) are abundant oligomeric enzymes increasingly recognized as important in the regulation of intracellular peroxide levels. Prx of the 2-Cys type have a peroxidatic (P) cysteine that become sulfenylated by a peroxide. The resulting sulfenic acid (-SOH) on the peroxidatic cysteine, condenses with the resolving (R) cysteine, generating a disulfide (-S-S-). Alternatively, the sulfenylated Prx can be oxidized further by reacting with a second peroxide to generate the sulfinic form (-SOOH) on the peroxidatic cysteine. Sulfiredoxin (Srx) can reverse this overoxidation by reducing the Prx-SOOH back to Prx-SOH in an ATP-dependent reaction. Thioredoxin (Trx) use a thiol-disulfide exchange reaction that reduces a protein disulfide on Prx to generate two thiols (the starting Prx form in this illustration) while two cysteines in the Trx molecule are themselves oxidized to a disulfide. The regeneration of Trx is effected by thioredoxin reductase (TrxR), which like GPx, is a selenocysteine-bearing enzyme that uses NADPH rather than GSH as a reductant. Glutathione reductase (GR) also uses NADPH to reduce GSSG back to GSH. Thus, both the thioredoxin and glutathione systems utilize NADPH derived from the pentose phosphate pathway (PPP), and are ultimately dependent on the availability of glucose as an energy source.