Figure 1.
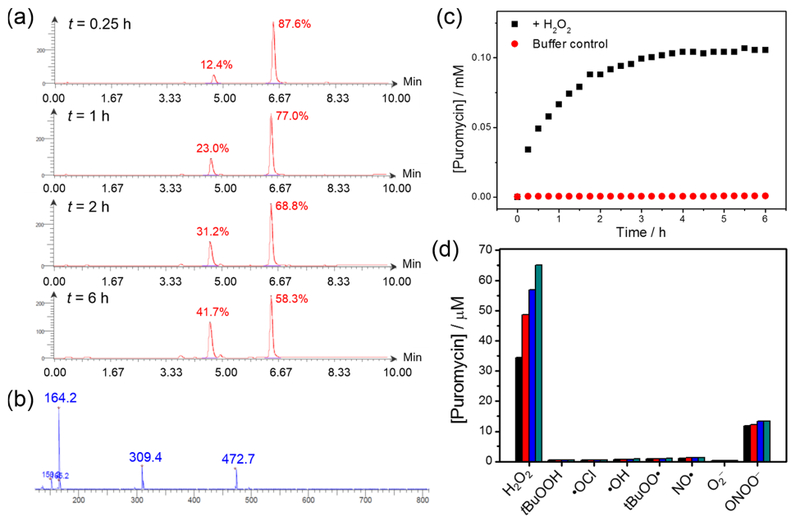
(a) LC chromatograms of the reaction between Peroxymycin-1 (0.3 mM) and H2O2 (0.1 mM) in phosphate buffer (20 mM, pH 7.4)/methanol solution mixture (2:1 v/v) with 3 vol % dimethyl sulfoxide (DMSO) at different time intervals. (b) MS of the peak with retention time 4.62 min, confirming formation of puromycin from the reaction between Peroxymycin-1 and H2O2. (c) Generation of puromycin from the solution of Peroxymycin-1, with and without H2O2, over time. (d) Selectivity of Peroxymycin-1 toward H2O2 over other reactive oxygen and nitrogen species. All species were at 100 μM except ONOO− was at 50 μM. Time points were taken at 15 min (black bars), 30 min (red bars), 45 min (blue bars), and 60 min (green bars).
