Abstract
Previous studies have suggested an increased risk of amyotrophic lateral sclerosis (ALS) and other motor neuron diseases for persons in occupations commonly involving exposure to diesel exhaust (DE). In this study, we investigated the association between occupational exposure to DE and odds of ALS. ALS cases were identified from the Danish National Patient Registry (1982–2013) and individually matched to 100 controls per case on the basis of birth year and sex. Using information on occupational history from 1964 onward obtained from the Danish Pension Fund, we estimated cumulative DE exposures using a job exposure matrix. We evaluated associations using conditional logistic regression analyses and stratified the analyses by sex. Using a 10-year lag period, DE exposure was positively associated with ALS among men who had ever been exposed (adjusted odds ratio (aOR) = 1.20, 95% confidence interval (CI): 1.05, 1.38). For men with greater than 50% probability of DE exposure, we observed a positive association between ALS and highest-quartile exposure during the 5-year (aOR = 1.35, 95% CI: 1.07, 1.70) and 10-year (aOR = 1.41, 95% CI: 1.11, 1.79) lag periods. Our study suggests an association between consistently higher exposures to DE and ALS in men, but not in women. These findings support previous reports of associations between ALS and occupations commonly involving DE exposure.
Keywords: ALS, amyotrophic lateral sclerosis, diesel exhaust, motor neuron disease, occupational exposure
As a progressively paralytic neurodegenerative disease, amyotrophic lateral sclerosis (ALS) has a notably brief average survival time of 3–5 years (1). Reports from the United States and Europe indicate an annual incidence of 1–2 new ALS cases per 100,000 people (2–4). Although approximately 10% of ALS cases are attributed to genetic inheritance (5), about 90% of ALS cases are sporadic. Overall the male:female ratio is skewed towards men (1), but more recently this ratio has been approaching unity (6, 7). Generally, the etiology of ALS is not well understood, but some researchers have suggested that preexisting genetic risk may be influenced by environmental exposures (1, 6, 8).
Some lines of evidence suggest that exposure to diesel exhaust (DE) might be a risk factor for ALS. Several studies have linked various components of DE, including hexane (9) and formaldehyde (10–13), to ALS, although 1 only suggested an association with formaldehyde (9) and 1 other study found no association (14). Many occupational studies, though not all (7, 15), have found increased risk of ALS among persons in occupations with high exposure to DE, such as truck drivers (16, 17), construction workers (9), machine operators (18), bus drivers (18), and military servicemen (19).
The observed genotoxicity resulting from DE exposure (20), along with the proposed associations of mutations and polymorphisms (1, 21) and oxidative stress (22, 23) with ALS, suggests biological plausibility of a relationship between DE exposure and ALS. Despite these lines of evidence leading to the hypothesis that DE exposure may be a risk factor for ALS and 1 study of occupations in which the investigators theorized a link with DE (17), no study, to our knowledge, has directly assessed the association between an estimate of DE exposure specifically and ALS. In this study, we used a job exposure matrix (JEM) to investigate the relationship between occupational DE exposure and ALS in a case-control study nested within the entire population of Denmark, using data from nationwide Danish registries.
METHODS
Study participants
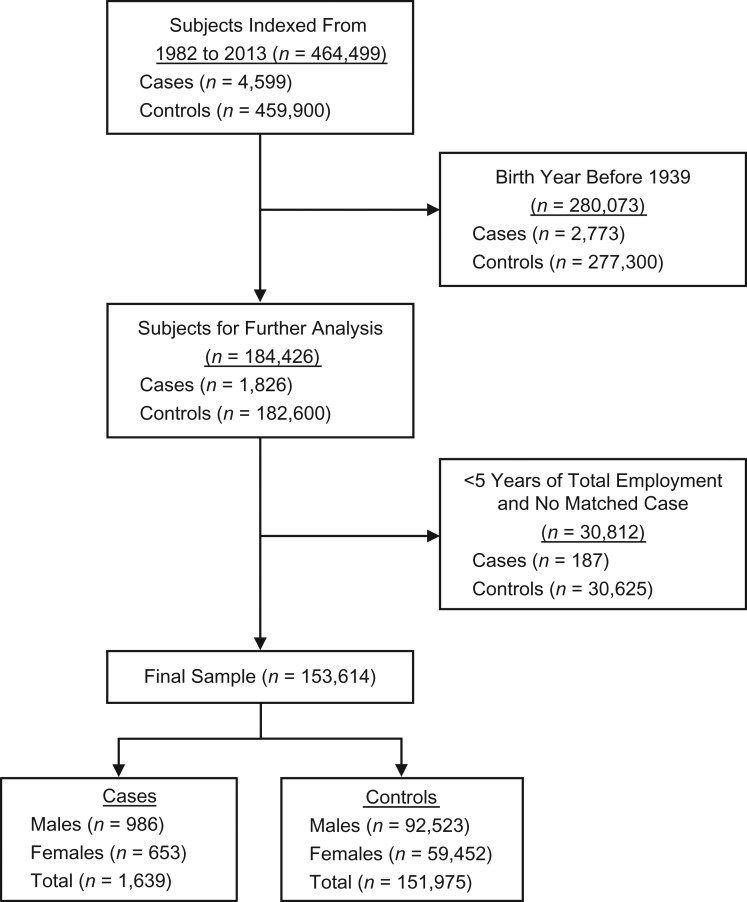
We identified ALS cases via International Classification of Diseases, Eighth Revision (pre-1994) and International Classification of Diseases, Tenth Revision (1994 and after) codes acquired from patient records included in the Danish National Patient Registry from its inception in 1977 (24, 25) through 2013. The Danish National Patient Registry originally included only inpatient data; outpatient data were later added, beginning in 1995 (24). Patients with a primary discharge diagnosis of amyotrophic lateral sclerosis (International Classification of Diseases, Eighth Revision, code 348.0) or motor neuron disease (International Classification of Diseases, Tenth Revision, code G12.2) were designated as ALS cases. The date of the first recorded ALS diagnosis was defined as the index date. The primary diagnosis in the Danish National Patient Registry is designated as the diagnosis associated with the initial hospital visit, while the secondary diagnosis indicates other diseases that may be underlying causes for the primary diagnosis (24). For each case, records for 100 individually birth-year- and sex-matched controls who were alive on the case’s index date were randomly selected using the Danish Central Person Register (26), which was founded in 1968 and keeps track of vital status, including dates of death and emigration, and assigned the same index date. Because the Danish National Patient Registry was created in 1977 (24, 25), we limited our analysis to persons who had a first recorded diagnosis on January 1, 1982, or later, to exclude potential prevalent cases (see Figure 1).
Figure 1.
Process of study subject selection and exclusion for analyses of diesel exhaust exposure and amyotrophic lateral sclerosis, Denmark, 1982–2013.
Exposure assessment
We used unique residential Central Person Register numbers to link the above-mentioned diagnosis and demographic data to the Danish Pension Fund, which has maintained data on the employment history of all residents of Denmark aged 16–66 years since April 1, 1964 (27). Employment records were based on 8-digit employer tax identification numbers indicating the companies for which study participants worked and 5-digit industry codes from an extended version of the International Standard Industrial Classification codes compiled by Statistics Denmark (more detailed codes introduced after 1992 were recoded to the original International Standard Industrial Classification codes for comparability) (27). Each company in Denmark is assigned a specific industry code. In some cases these codes are relatively descriptive for larger groups of occupational exposures (e.g., service station attendants, miners and quarrymen, railway engine and truck drivers); others (e.g., general public services) are relatively broad.
For this study, we used a JEM constructed for Denmark by investigators in the Nordic Occupational Cancer Study, for which methods for development have already been reported (28). In summary, the Finnish version of this JEM was modified by one of the authors (J.H.) for relevance to the population of Denmark on the basis of industrial measurements of DE from Finland and Denmark. The expected measurements in the JEM used in this study are also time-specific, with time periods of 1960–1974, 1975–1984, and after 1984 for probability and intensity (mg/m3) of exposure for each of the 5-digit industry codes (see Web Table 1, available at https://academic.oup.com/aje, for the list of industries with DE exposure). Using this DE JEM, we found an association between DE and chronic obstructive pulmonary disease similar to what has previously been reported (Web Appendix).
In our initial analysis, we calculated time-specific exposure by multiplying the probability (range, 0–0.90) and intensity (range, 0–0.88 mg/m3) of exposure associated with each industry in which a subject worked (28). The results were then multiplied by the duration of time spent working in specific occupations and summed to determine the cumulative expected exposure of each participant. In secondary analyses, we limited exposure calculations to occupations in industries with at least a 50% probability of exposure, with persons having a <50% probability of exposure designated as unexposed. We then calculated exposure by multiplying the intensity of exposure for each industry by the number of days employed in each occupation to explore an exposure variable focused more on intensity of exposure. Total length of employment in diesel-exposed industries ranged from 5 days to 3,926 days (10.7 years), with cases working an average of 2,123 days (5.8 years) and controls an average of 2,074 days (5.7 years).
We also explored 5- and 10-year exposure lag periods before the index date (i.e., excluding exposures that occurred within those time periods) to exclude exposures that could have occurred during any time of undiagnosed ALS, examine possible variations in associations due to timing, and mitigate potential healthy-worker survivor bias. Furthermore, to diminish exposure misclassification and potential left-truncation bias as a result of work performed prior to the creation of the Danish Pension Fund in 1964 (29), we excluded study subjects who were older than age 25 years in 1964 (i.e., born in 1940 or earlier) (26). Additionally, in an attempt to avoid healthy-worker hire bias, participants with less than 5 years of total work experience (short-term employment) were removed from the analysis (30). Cases and controls with no matches were also removed from the analysis. The process of exclusions made to arrive at the final analytical study sample is presented in Figure 1.
Covariates
Covariates in adjusted analyses included socioeconomic status (SES) and residential area on the index date. SES was categorized into 5 ordered groups based on tax-recorded occupational title: 1) academics and corporate managers; 2) people with high-salary positions (entrepreneurs, managers, and teachers); 3) people with low-salary positions (nurses and technicians); 4) skilled workers; and 5) unskilled workers. SES for married subjects was based on the highest SES of the participant or spouse. SES was designated as “unknown” when individuals were suspected to be unemployed (unemployment was suspected because neither the participant nor the spouse had a tax-recorded job title in the Central Person Register at that time). Categories for area of residence included Copenhagen (capital), Copenhagen suburbs, Aarhus/Odense, provincial towns, rural areas, and Greenland. Information for the covariates was obtained from the Central Person Register and updated on a daily basis. Values that were unknown for covariates were set to “missing” for analyses.
Statistical analysis
We used conditional logistic regression to obtain odds ratios and 95% confidence intervals. ALS cases and controls were classified as ever or never exposed to DE. Additionally, expected cumulative exposure (or our intensity measure) to DE greater than zero was categorized into quartiles based on the distribution in controls for 10-year-lagged exposures, which was determined to have better fit than a model with the continuous exposure measure according to the Akaike Information Criterion; persons with no exposure served as the reference group. For comparability, we kept the same exposure categories for the other lag periods. These models adjusted for SES categories and geographic location; age was accounted for in the matching process. Because of likely differences in exposure assignment with the JEM by sex—due to differences in jobs and tasks performed by men and women in the same industry, especially in earlier time periods—we stratified the analyses by sex. We conducted linear trend tests using continuous DE exposure and intensity measures.
All analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina) (31). Due to the secondary nature of this analysis, the requirement of informed consent was waived. The study was exempt from full review by the institutional review board of the Harvard T.H. Chan School of Public Health and was approved by the Danish Data Protection Agency.
RESULTS
The demographic characteristics of our analytical study population by DE exposure are displayed in Table 1. We analyzed data for 1,639 ALS cases and 151,975 controls. Among males, large portions of both ALS cases and controls had been occupationally exposed to DE (85% and 83%, respectively). With regard to socioeconomic categories, the greatest proportions of DE-exposed ALS cases (34%) and controls (35%) belonged to the “skilled workers” group.
Table 1.
Characteristics of Amyotrophic Lateral Sclerosis Cases (n = 1,639) and Matched Controls (n = 151,975) on the Index Date, by Diesel Exhaust Exposure, Denmark, 1982–2013
| Characteristic | Controls | Cases | ||||||
|---|---|---|---|---|---|---|---|---|
| Unexposed to DE (n = 95,440) | Exposed to DE (n = 56,535) | Unexposed to DE (n = 1,041) | Exposed to DE (n = 598) | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Male sex | 45,712 | 47.90 | 46,811 | 82.80 | 480 | 46.11 | 506 | 84.62 |
| Age, years | ||||||||
| <45 | 11,357 | 11.90 | 6,878 | 12.17 | 145 | 13.93 | 59 | 9.87 |
| 45–54 | 23,975 | 25.12 | 15,025 | 26.58 | 244 | 23.44 | 172 | 28.76 |
| 55–64 | 38,798 | 40.65 | 23,316 | 41.24 | 421 | 40.44 | 243 | 40.64 |
| 65–74 | 21,310 | 22.33 | 11,316 | 20.02 | 231 | 22.19 | 124 | 20.74 |
| Socioeconomic statusa | ||||||||
| Academics and managers | 12,651 | 13.26 | 4,659 | 8.24 | 153 | 14.70 | 50 | 8.36 |
| High-salary positions | 15,019 | 15.74 | 7,351 | 13.00 | 166 | 15.95 | 77 | 12.88 |
| Low-salary positions | 18,557 | 19.44 | 8,775 | 15.52 | 202 | 19.40 | 105 | 17.56 |
| Skilled workers | 27,707 | 29.03 | 19,727 | 34.89 | 280 | 26.90 | 203 | 33.95 |
| Unskilled workers | 12,609 | 13.21 | 11,162 | 19.74 | 139 | 13.35 | 118 | 19.73 |
| Unknown | 8,897 | 9.32 | 4,861 | 8.60 | 101 | 9.70 | 45 | 7.53 |
| Residence at diagnosis/index date | ||||||||
| Copenhagen | 9,882 | 10.35 | 5,219 | 9.23 | 112 | 10.76 | 58 | 9.70 |
| Copenhagen suburbs | 23,045 | 24.15 | 13,649 | 24.14 | 251 | 24.11 | 142 | 23.75 |
| Aarhus/Odense | 9,520 | 9.97 | 5,405 | 9.56 | 103 | 9.89 | 42 | 7.02 |
| Provincial towns | 38,910 | 40.77 | 23,007 | 40.70 | 441 | 42.36 | 259 | 43.31 |
| Rural areas | 13,815 | 14.48 | 8,999 | 15.92 | 132 | 12.68 | 92 | 15.38 |
| Greenland | 67 | 0.07 | 69 | 0.12 | 0 | 0.00 | 2 | 0.33 |
| Unknown | 201 | 0.21 | 187 | 0.33 | 2 | 0.19 | 3 | 0.50 |
| Marital status | ||||||||
| Married | 65,745 | 68.89 | 36,837 | 65.16 | 718 | 68.97 | 403 | 67.39 |
| Unmarried | 11,570 | 12.12 | 8,359 | 14.79 | 115 | 11.05 | 82 | 13.71 |
| Divorced | 12,771 | 13.38 | 9,166 | 16.21 | 146 | 14.02 | 94 | 15.72 |
| Widowed | 5,179 | 5.43 | 2,097 | 3.71 | 59 | 5.67 | 19 | 3.18 |
| Unknown | 175 | 0.18 | 76 | 0.13 | 3 | 0.29 | 0 | 0 |
| Cumulative estimated DE exposure, mg/m3 b | ||||||||
| No lag | 65.19 (13.79–208.65) | 67.75 (14.20–217.26) | ||||||
| 5-year lag | 40.78 (10.00–128.57) | 43.44 (10.13–130.31) | ||||||
| 10-year lag | 39.28 (9.73–122.72) | 40.81 (9.54–126.73) | ||||||
Abbreviation: DE, diesel exhaust.
a Where a spouse’s job title was available, socioeconomic status was based on the highest status of the study participant or his/her spouse.
b Values are expressed as median (interquartile range).
In our analysis of occupational DE exposure among males in our study population, the effect estimate for ever exposure increased with increasing exposure lags from none to 10 years (Table 2). The adjusted odds ratio for ALS among men who had ever experienced occupational DE exposure at least 10 years prior to the index date was 1.20 (95% confidence interval (CI): 1.05, 1.38). Additionally, when we limited exposures to industries in which men were employed at least 10 years prior to the index date, the adjusted odds ratio increased by quartile of exposure; the adjusted odds ratio for the lowest quartile of exposure (<11.55 mg/m3) was 1.23 (95% CI: 1.03, 1.50), although the overall trend was not significant (aOR = 1.08, 95% CI: 0.99, 1.19). No such associations appeared among women (Table 3). Although risk was significantly decreased among women in the second quartile in the analysis of 5-year-lagged exposure, there was no obvious trend, and results did not fit any clear pattern.
Table 2.
Odds of Amyotrophic Lateral Sclerosis According to Cumulative Exposure to Diesel Exhaust Among Males (n = 93,509), Denmark, 1982–2013
| Lag and Exposure Level | Controls (n = 92,523) | Cases (n = 986) | ORa | 95% CI | aORb | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| No lag | ||||||||
| No exposure | 45,712 | 49.41 | 480 | 48.68 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 c | 46,811 | 50.59 | 506 | 51.32 | 1.09 | 0.96, 1.24 | 1.13 | 0.99, 1.29 |
| <11.55 | 9,331 | 10.09 | 106 | 10.75 | 1.08 | 0.88, 1.34 | 1.08 | 0.87, 1.33 |
| 11.55–45.62 | 9,293 | 10.04 | 94 | 9.53 | 0.97 | 0.77, 1.21 | 0.98 | 0.78, 1.22 |
| 45.63–135.40 | 10,263 | 11.09 | 107 | 10.85 | 1.00 | 0.81, 1.23 | 1.02 | 0.82, 1.26 |
| ≥135.41 | 17,924 | 19.37 | 199 | 20.18 | 1.06 | 0.90, 1.26 | 1.10 | 0.93, 1.30 |
| Test for trendd | 1.01 | 0.98, 1.04 | 1.02 | 0.99, 1.05 | ||||
| 5-year lag | ||||||||
| No exposure | 50,177 | 54.23 | 515 | 52.23 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 42,346 | 45.77 | 471 | 47.77 | 1.10 | 0.97, 1.25 | 1.14 | 0.99, 1.30 |
| <11.55 | 10,259 | 11.09 | 123 | 12.47 | 1.17 | 0.97, 1.44 | 1.15 | 0.93, 1.42 |
| 11.55–45.62 | 10,369 | 11.21 | 106 | 10.75 | 1.00 | 0.81, 1.24 | 1.04 | 0.83, 1.30 |
| 45.63–135.40 | 10,531 | 11.38 | 120 | 12.17 | 1.12 | 0.92, 1.37 | 1.17 | 0.94, 1.43 |
| ≥135.41 | 11,187 | 12.09 | 122 | 12.37 | 1.07 | 0.88, 1.31 | 1.16 | 0.95, 1.43 |
| Test for trend | 1.02 | 0.97, 1.07 | 1.03 | 0.98, 1.09 | ||||
| 10-year lag | ||||||||
| No exposure | 52,569 | 56.82 | 525 | 53.25 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 39,954 | 43.18 | 461 | 46.75 | 1.17 | 1.03, 1.33 | 1.20 | 1.05, 1.38 |
| <11.55 | 9,989 | 10.80 | 121 | 12.27 | 1.23 | 1.01, 1.50 | 1.23 | 1.03, 1.50 |
| 11.55–45.62 | 9,990 | 10.80 | 109 | 11.05 | 1.11 | 0.90, 1.36 | 1.12 | 0.91, 1.39 |
| 45.63–135.40 | 9,987 | 10.79 | 115 | 11.66 | 1.17 | 0.95, 1.43 | 1.20 | 0.97, 1.47 |
| ≥135.41 | 9,988 | 10.80 | 116 | 11.76 | 1.18 | 0.96, 1.44 | 1.22 | 0.99, 1.50 |
| Test for trend | 1.03 | 0.99, 1.09 | 1.08 | 0.99, 1.19 | ||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
a Controls were individually matched to cases on age and sex.
b Models adjusted for socioeconomic status and residential location.
c Cumulative exposure = [(level of exposure) × (probability of exposure)/100] × number of days employed.
d Tests for trend were conducted per 100-mg/m3 increment of diesel exhaust exposure.
Table 3.
Odds of Amyotrophic Lateral Sclerosis According to Cumulative Exposure to Diesel Exhaust Among Females (n = 60,105), Denmark, 1982–2013
| Lag and Exposure Level | Controls (n = 59,452) | Cases (n = 653) | ORa | 95% CI | aORb | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| No lag | ||||||||
| No exposure | 49,728 | 83.64 | 561 | 85.91 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 c | 9,724 | 16.36 | 92 | 14.09 | 0.85 | 0.68, 1.06 | 0.82 | 0.65, 1.03 |
| <4.58 | 2,111 | 3.55 | 19 | 2.91 | 0.80 | 0.51, 1.27 | 0.78 | 0.48, 1.27 |
| 4.58–17.51 | 2,152 | 3.62 | 14 | 2.14 | 0.58 | 0.34, 0.99 | 0.55 | 0.30, 0.96 |
| 17.52–64.57 | 2,322 | 3.91 | 26 | 3.98 | 1.00 | 0.67, 1.48 | 0.95 | 0.63, 1.45 |
| ≥64.58 | 3,139 | 5.28 | 33 | 5.05 | 0.94 | 0.66, 1.34 | 0.93 | 0.64, 1.34 |
| Test for trendd | 0.99 | 0.90, 1.10 | 1.00 | 0.90, 1.10 | ||||
| 5-year lag | ||||||||
| No exposure | 51,009 | 85.80 | 570 | 87.29 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 8,443 | 14.20 | 83 | 12.71 | 0.89 | 0.71, 1.12 | 0.84 | 0.66, 1.08 |
| <4.58 | 2,074 | 3.49 | 18 | 2.76 | 0.79 | 0.49, 1.26 | 0.76 | 0.46, 1.25 |
| 4.58–17.51 | 2,129 | 3.58 | 16 | 2.45 | 0.68 | 0.41, 1.12 | 0.58 | 0.34, 1.01 |
| 17.52–64.57 | 2,097 | 3.53 | 25 | 3.83 | 1.08 | 0.72, 1.61 | 1.06 | 0.70, 1.61 |
| ≥64.58 | 2,143 | 3.60 | 24 | 3.68 | 1.01 | 0.67, 1.51 | 0.98 | 0.64, 1.49 |
| Test for trend | 1.08 | 0.95, 1.24 | 1.09 | 0.95, 1.24 | ||||
| 10-year lag | ||||||||
| No exposure | 51,716 | 86.99 | 578 | 88.51 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 7,736 | 13.01 | 75 | 11.49 | 0.88 | 0.69, 1. 12 | 0.84 | 0.65, 1.08 |
| <4.58 | 1,929 | 3.24 | 18 | 2.76 | 0.85 | 0.53, 1.3 | 0.80 | 0.49, 1.32 |
| 4.58–17.51 | 1,939 | 3.26 | 13 | 1.99 | 0.61 | 0.35, 1.05 | 0.54 | 0.30, 0.98 |
| 17.52–64.57 | 1,934 | 3.25 | 24 | 3.68 | 1.12 | 0.74, 1.69 | 1.08 | 0.71, 1.67 |
| ≥64.58 | 1,934 | 3.25 | 20 | 3.06 | 0.94 | 0.60, 1.47 | 0.92 | 0.58, 1.47 |
| Test for trend | 1.08 | 0.93, 1.24 | 1.08 | 0.93, 1.24 | ||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
a Controls were individually matched to cases on age and sex.
b Models adjusted for socioeconomic status and residential location.
c Cumulative exposure = [(level of exposure) × (probability of exposure)/100] × number of days employed.
d Tests for trend were conducted per 100-mg/m3 increment of diesel exhaust exposure.
Results from our analysis of DE intensity in males, assigning industries with less than a 50% probability of DE exposure as involving no exposure, are shown in Table 4. The results showed overall larger effect estimates than those from analyses with cumulative expected exposure, but the pattern of increasing effect estimates with longer exposure lags was also present. The adjusted odds ratio for ever working in an industry with more than a 50% probability of DE exposure was 1.10 (95% CI: 0.95, 1.26) for no exposure lag, 1.16 (95% CI: 1.01, 1.35) for a 5-year lag, and 1.19 (95% CI: 1.03, 1.38) for a 10-year lag. Additionally, men with the highest quartile measurement of cumulative DE exposure intensity in the 10-year-lag analyses (≥141.96 mg/m3) had 41% increased odds of ALS in adjusted analysis (adjusted odds ratio (aOR) = 1.41, 95% CI: 1.11, 1.79). The overall trends for 5-year and 10-year lags were also significant (aOR = 1.05 (95% CI: 1.01, 1.10) and aOR = 1.05 (95% CI: 1.00, 1.09), respectively). Among women, there was again no obvious pattern of association with DE; the adjusted odds ratios were slightly elevated in the highest exposure categories but not statistically significant (Table 5).
Table 4.
Odds of Amyotrophic Lateral Sclerosis According to Intensity of Cumulative Diesel Exhaust Exposure Among Males (n = 93,509), Denmark, 1982–2013
| Lag and Exposure Level | Controls (n = 92,523) | Cases (n = 986) | ORa | 95% CI | aORb | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| No lag | ||||||||
| No exposure | 66,109 | 71.45 | 690 | 69.98 | 1.00 | Referent | Referent | |
| Ever exposure, mg/m3 c | 26,414 | 28.55 | 296 | 30.02 | 1.08 | 0.94, 1.24 | 1.10 | 0.95, 1.26 |
| <11.55 | 5,642 | 6.10 | 70 | 7.10 | 1.19 | 0.93, 1.53 | 1.19 | 0.93, 1.52 |
| 11.55–42.30 | 5,514 | 5.96 | 51 | 5.17 | 0.89 | 0.67, 1.18 | 0.90 | 0.68, 1.20 |
| 42.31–141.60 | 5,728 | 6.19 | 58 | 5.88 | 0.97 | 0.74, 1.27 | 1.00 | 0.76, 1.31 |
| ≥141.61 | 9,530 | 10.30 | 117 | 11.87 | 1.18 | 0.97, 1.44 | 1.22 | 1.00, 1.50 |
| Test for trendd | 1.01 | 0.99, 1.03 | 1.01 | 0.99, 1.04 | ||||
| 5-year lag | ||||||||
| No exposure | 69,023 | 74.60 | 714 | 72.41 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 23,500 | 26.68 | 272 | 27.59 | 1.13 | 0.98, 1.30 | 1.16 | 1.01, 1.35 |
| <11.55 | 5,747 | 6.21 | 72 | 7.30 | 1.22 | 0.96, 1.56 | 1.22 | 0.95, 1.60 |
| 11.55–42.30 | 5,715 | 6.18 | 54 | 5.48 | 0.92 | 0.70, 1.21 | 0.94 | 0.71, 1.24 |
| 42.31–141.60 | 5,833 | 6.30 | 63 | 6.39 | 1.05 | 0.81, 1.36 | 1.08 | 0.83, 1.40 |
| ≥141.61 | 6,205 | 6.71 | 83 | 8.42 | 1.30 | 1.03, 1.64 | 1.35 | 1.07, 1.70 |
| Test for trend | 1.04 | 1.00, 1.09 | 1.05 | 1.01, 1.10 | ||||
| 10-year lag | ||||||||
| No exposure | 70,580 | 76.28 | 725 | 73.53 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 21,943 | 23.72 | 261 | 26.47 | 1.17 | 1.01, 1.35 | 1.19 | 1.03, 1.38 |
| <11.55 | 5,491 | 5.93 | 68 | 6.90 | 1.22 | 0.95, 1.56 | 1.22 | 0.95, 1.56 |
| 11.55–42.30 | 5,481 | 5.92 | 53 | 5.38 | 0.95 | 0.72, 1.26 | 0.97 | 0.73, 1.28 |
| 42.31–141.60 | 5,484 | 5.93 | 64 | 6.49 | 1.15 | 0.89, 1.48 | 1.18 | 0.91, 1.53 |
| ≥141.61 | 5,487 | 5.93 | 76 | 7.71 | 1.36 | 1.07, 1.73 | 1.41 | 1.11, 1.79 |
| Test for trend | 1.04 | 1.00, 1.09 | 1.05 | 1.00, 1.09 | ||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
a Controls were individually matched to cases on age and sex.
b Models adjusted for socioeconomic status and residential location.
c Cumulative exposure intensity = level of exposure × number of days employed in jobs with a >50% probability of exposure.
d Tests for trend were conducted per 100-mg/m3 increment of diesel exhaust exposure.
Table 5.
Odds of Amyotrophic Lateral Sclerosis According to Intensity of Cumulative Diesel Exhaust Exposure Among Females (n = 60,105), Denmark, 1982–2013
| Lag and Exposure Level | Controls (n = 59,452) | Cases (n = 653) | ORa | 95% CI | aORb | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| No lag | ||||||||
| No exposure | 55,882 | 94.00 | 613 | 93.87 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 c | 3,570 | 6.00 | 40 | 6.13 | 1.03 | 0.75, 1.42 | 1.04 | 0.75, 1.43 |
| <8.36 | 764 | 1.29 | 6 | 0.92 | 0.72 | 0.32, 1.61 | 0.67 | 0.28, 1.61 |
| 8.36–29.81 | 754 | 1.27 | 10 | 1.53 | 1.22 | 0.65, 2.29 | 1.31 | 0.70, 2.46 |
| 29.82–100.42 | 818 | 1.38 | 6 | 0.92 | 0.68 | 0.30, 1.51 | 0.61 | 0.25, 1.47 |
| ≥100.43 | 1,234 | 2.08 | 18 | 2.76 | 1.34 | 0.84, 2.15 | 1.41 | 0.88, 2.26 |
| Test for trendd | 1.01 | 0.94, 1.08 | 1.01 | 0.94, 1.08 | ||||
| 5-year lag | ||||||||
| No exposure | 56,457 | 94.96 | 621 | 95.10 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 2,995 | 5.04 | 32 | 4.90 | 0.98 | 0.69, 1.40 | 0.99 | 0.69, 1.42 |
| <8.36 | 742 | 1.25 | 5 | 0.77 | 0.62 | 0.26, 1.50 | 0.63 | 0.26, 1.51 |
| 8.36–29.81 | 748 | 1.26 | 8 | 1.23 | 0.98 | 0.49, 1.98 | 0.99 | 0.49, 2.00 |
| 29.82–100.42 | 744 | 1.25 | 8 | 1.23 | 0.99 | 0.49, 1.99 | 1.00 | 0.50, 2.01 |
| ≥100.43 | 761 | 1.28 | 11 | 1.68 | 1.33 | 0.73, 1.99 | 1.33 | 0.73, 2.43 |
| Test for trend | 1.10 | 0.97, 1.25 | 1.10 | 0.97, 1.25 | ||||
| 10-year lag | ||||||||
| No exposure | 56,707 | 95.38 | 623 | 95.41 | 1.00 | Referent | 1.00 | Referent |
| Ever exposure, mg/m3 | 2,745 | 4.62 | 30 | 4.59 | 1.00 | 0.70, 1.45 | 1.01 | 0.70, 1.47 |
| <8.36 | 687 | 1.16 | 5 | 0.77 | 0.67 | 0.28, 1.62 | 0.63 | 0.26, 1.51 |
| 8.36–29.81 | 683 | 1.15 | 8 | 1.23 | 1.08 | 0.53, 2.17 | 0.99 | 0.49, 2.00 |
| 29.82–100.42 | 689 | 1.16 | 8 | 1.23 | 1.07 | 0.53, 2.15 | 1.08 | 0.53, 2.18 |
| ≥100.43 | 686 | 1.15 | 9 | 1.38 | 1.20 | 0.63, 2.33 | 1.20 | 0.62, 2.35 |
| Test for trend | 1.10 | 0.96, 1.27 | 1.10 | 0.96, 1.27 | ||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
a Controls were individually matched to cases on age and sex.
b Models adjusted for socioeconomic status and residential location.
c Cumulative exposure intensity = level of exposure × number of days employed in jobs with a >50% probability of exposure.
d Tests for trend were conducted per 100-mg/m3 increment of diesel exhaust exposure.
DISCUSSION
In our study of ALS cases diagnosed in Denmark from 1982 to 2013, we found an association between occupational DE exposure and odds of ALS in men. The results were stronger with longer exposure lags, which could relate to either a reduction in healthy-worker survivor bias with increasing exposure lag times (32) or the possibility that the relevant time window of exposure for the influence of DE on ALS risk is many years prior to ALS clinical onset. We did not see any associations among women, other than reduced odds in the second quartile of the 10-year-lagged analysis of cumulative expected DE exposure, which most likely was a chance finding. Previous mouse models of neurodegeneration have indicated sex differences and sex-dependent susceptibility to neurotoxicity from air pollution exposures (33, 34). Other than a difference in underlying biological response to DE exposure, it is likely that the types of jobs and tasks men and women perform in a given industry may differ. This would lead to the JEM not capturing exposures among men and women in the same way, which also would produce differences by sex. However, we had many fewer exposed female cases than male cases, which could also have contributed to the differences seen. Future studies with more exposed women are warranted.
Diesel engines are used in a variety of machinery and transport vehicles (35), with diesel-powered cars being highly popular in European countries due to their fuel efficiency (36). DE is composed of several toxic gaseous and particulate compounds, including carbon dioxide (CO2), carbon monoxide (CO), nitrogen dioxide (NO2), elemental carbon (C), and sulfur dioxide (SO2) (35), and has been linked directly and indirectly to various adverse health outcomes (37). It is a well-known irritant and carcinogen (38), and it has also been found to be positively associated with lung cancer (39, 40), chronic obstructive pulmonary disease (41), and adverse cardiovascular events (42, 43).
Air pollution has been implicated as a risk factor for brain inflammation and neurodegenerative disorders (33, 44–46). With DE being a major component of traffic-related air pollution, it has been suggested that this particular air pollutant could be related to the etiology of ALS (17, 45). Specifically, the ability of DE to influence oxidative stress has been implicated as a potential mechanism for neurotoxicity and subsequent degeneration (22, 23, 33, 45). Furthermore, cigarette smoking has genotoxic properties that have been suggested to underlie observed associations with ALS (47), and DE has genotoxic properties that are similar to those of cigarette smoking (20). Several studies have investigated associations between employment in certain occupations and risk of ALS (11, 48–52). Many investigators have reported positive associations with ALS and working in occupations that can have a high probability of DE exposure, including driving buses and trucks (16–18), working in construction (9, 48), farming (15, 49, 53), operating machinery (15, 18), and serving in the military (19, 48, 54, 55). However, our study is the first to have specifically targeted DE exposure, by using a JEM with prospectively collected information on occupations.
Use of a JEM to estimate subjects’ exposures to DE before ALS diagnoses allows for a more individualized marker of cumulative exposure than simply relating different occupations to ALS. Additionally, the objective collection of occupational data prospectively through the registry is probably better than self-reported occupational history, although whether assigning exposures based on industry groupings rather than self-reported specific jobs and tasks is more accurate is not clear. However, the ability to have each subject’s full employment history certainly allows for a better estimate of cumulative exposure at different times prior to a possible ALS diagnosis than relying only on the longest-held occupation or the job held at 1 specific time point, as in several previous studies.
Despite the strength of using prospectively collected occupational data to estimate cumulative DE exposure prior to ALS diagnosis using individual exposure estimates, there were some limitations to this study. We did not have information on the smoking status of participants in this study; thus, we could not adjust for smoking as a potential confounder. However, smoking was common among men in Denmark at the time most of these subjects were exposed to DE; prevalence was more than 70% in the 1960s but had declined to slightly less than 30% by 2010 (56). There would have to have been a higher prevalence of smoking among persons working in DE-exposed jobs to explain any observed increase in ALS risk due to smoking (57). Additionally, considering that SES in Denmark has been correlated with smoking habits (58), we may have indirectly adjusted for smoking status by adjusting for SES in our analyses. In addition, while there is evidence that smoking is related to risk of ALS (59–62), that increased risk may be more prominent among women than among men (63, 64). Given that our current results were essentially only among men, this argues somewhat against smoking’s accounting for our findings (65).
Because ALS diagnosis was determined using both inpatient and outpatient hospital records, there was a small risk of ALS case-status misclassification (66). Such misclassification would have to have been strongly related to DE exposure to account for our findings, which we have no reason to suspect. In addition, because the employment history registry used for our analysis was created in 1964, we were unable to determine exposures for any jobs held before that time point. Thus, some exposure misclassification may have been present. However, there is no reason to suspect that such misclassification would have differed by case status and so, if anything, this would have likely biased our results towards the null. We also attempted to minimize such bias by restricting the analysis to persons who were 25 years of age or younger at the start of the occupational registry.
The Danish JEM used in our study was based on the template of a Finnish JEM, FINJEM (28), and Danish measurements of DE were used when available; if not, measurements were adopted from FINJEM and adjusted based on experts’ assessments. As Nordic countries, Denmark and Finland are in many ways very similar in terms of socioeconomic equality, including occupational exposure levels. Lastly, despite targeting DE exposure specifically, JEMs still involve measurement error relative to actual personal exposures. Some additional error of this sort may have been introduced because of changes in the Pension Fund codes after 1992, although the JEMs attempted to minimize this by recoding the more detailed later classifications into the broader earlier ones. These errors, however, were probably unrelated to ALS status (partly because information was collected prior to disease onset), and so they would also likely have biased our results towards the null, if anything.
We observed an association between DE exposure at least 10 years prior to index dates and a higher risk of ALS among males. These findings, particularly given the mutagenic potential of DE and the potential role of mutations (1, 67) and oxidative stress (23) in ALS, suggest that this is an exposure which warrants more attention in ALS etiology. Although our assessment was of occupational exposures, widespread population exposures to DE do occur, particularly from some traffic pollution, though most often at a lower level than the occupational exposures. Studies of exposure to DE in the general population are warranted. Given the widespread nature of DE exposure but the rarity of ALS, an association with DE could suggest that only certain people are sensitive to DE exposure, possibly determined by genetic profile.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Departments of Epidemiology and Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Aisha S. Dickerson, Marc G. Weisskopf); and Danish Cancer Society Research Center, Copenhagen, Denmark (Johnni Hansen, Ole Gredal).
This work was funded by the National Institute of Environmental Health Sciences, US National Institutes of Health (grants R01 ES019188 and P30 ES000002 to M.G.W.). A.S.D. was supported in part by a National Institutes of Health training grant (grant T32 ES007069).
Conflict of interest: none declared.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- aOR
adjusted odds ratio
- CI
confidence interval
- DE
diesel exhaust
- JEM
job exposure matrix
- SES
socioeconomic status
REFERENCES
- 1. Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377(2):162–172. [DOI] [PubMed] [Google Scholar]
- 2. Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14(4):248–264. [DOI] [PubMed] [Google Scholar]
- 3. Chiò A, Logroscino G, Traynor BJ, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seals RM, Hansen J, Gredal O, et al. Age-period-cohort analysis of trends in amyotrophic lateral sclerosis in Denmark, 1970–2009. Am J Epidemiol. 2013;178(8):1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulder DW, Kurland LT, Offord KP, et al. Familial adult motor neuron disease: amyotrophic lateral sclerosis. Neurology. 1986;36(4):511–517. [DOI] [PubMed] [Google Scholar]
- 6. Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617–628. [DOI] [PubMed] [Google Scholar]
- 7. Logroscino G, Traynor BJ, Hardiman O, et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry. 2008;79(1):6–11. [DOI] [PubMed] [Google Scholar]
- 8. Oskarsson B, Horton DK, Mitsumoto H. Potential environmental factors in amyotrophic lateral sclerosis. Neurol Clin. 2015;33(4):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang F, Quinlan P, Ye W, et al. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117(9):1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seals RM, Kioumourtzoglou MA, Gredal O, et al. Occupational formaldehyde and amyotrophic lateral sclerosis. Eur J Epidemiol. 2017;32(10):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters TL, Kamel F, Lundholm C, et al. Occupational exposures and the risk of amyotrophic lateral sclerosis. Occup Environ Med. 2017;74(2):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisskopf MG, Morozova N, O’Reilly EJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(5):558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts AL, Johnson NJ, Cudkowicz ME, et al. Job-related formaldehyde exposure and ALS mortality in the USA. J Neurol Neurosurg Psychiatry. 2016;87(7):786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinkerton LE, Hein MJ, Meyers A, et al. Assessment of ALS mortality in a cohort of formaldehyde-exposed garment workers. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(5-6):353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sutedja NA, Fischer K, Veldink JH, et al. What we truly know about occupation as a risk factor for ALS: a critical and systematic review. Amyotroph Lateral Scler. 2009;10(5–6):295–301. [DOI] [PubMed] [Google Scholar]
- 16. Kurtzke JF, Beebe GW. Epidemiology of amyotrophic lateral sclerosis: 1. A case-control comparison based on ALS deaths. Neurology. 1980;30(5):453–462. [DOI] [PubMed] [Google Scholar]
- 17. Pamphlett R, Rikard-Bell A. Different occupations associated with amyotrophic lateral sclerosis: is diesel exhaust the link? PLoS One. 2013;8(11):e80993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park RM, Schulte PA, Bowman JD, et al. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48(1):63–77. [DOI] [PubMed] [Google Scholar]
- 19. Seals RM, Kioumourtzoglou MA, Hansen J, et al. Amyotrophic lateral sclerosis and the military: a population-based study in the Danish registries. Epidemiology. 2016;27(2):188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steiner S, Bisig C, Petri-Fink A, et al. Diesel exhaust: current knowledge of adverse effects and underlying cellular mechanisms. Arch Toxicol. 2016;90(7):1541–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schymick JC, Talbot K, Traynor BJ. Genetics of sporadic amyotrophic lateral sclerosis. Hum Mol Genet. 2007;16(spec. no. 2):R233–R242. [DOI] [PubMed] [Google Scholar]
- 22. Simpson EP, Yen AA, Appel SH. Oxidative stress: a common denominator in the pathogenesis of amyotrophic lateral sclerosis. Curr Opin Rheumatol. 2003;15(6):730–736. [DOI] [PubMed] [Google Scholar]
- 23. D’Amico E, Factor-Litvak P, Santella RM, et al. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pedersen C, Poulsen AH, Rod NH, et al. Occupational exposure to extremely low-frequency magnetic fields and risk for central nervous system disease: an update of a Danish cohort study among utility workers. Int Arch Occup Environ Health. 2017;90(7):619–628. [DOI] [PubMed] [Google Scholar]
- 26. Pedersen CB, Gøtzsche H, Møller JO, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 27. Hansen J, Lassen CF. The Supplementary Pension Fund Register. Scand J Public Health. 2011;39(7 suppl):99–102. [DOI] [PubMed] [Google Scholar]
- 28. Kauppinen T, Heikkilä P, Plato N, et al. Construction of job-exposure matrices for the Nordic Occupational Cancer Study (NOCCA). Acta Oncol. 2009;48(5):791–800. [DOI] [PubMed] [Google Scholar]
- 29. Applebaum KM, Malloy EJ, Eisen EA. Left truncation, susceptibility, and bias in occupational cohort studies. Epidemiology. 2011;22(4):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buckley JP, Keil AP, McGrath LJ, et al. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology. 2015;26(2):204–212. [DOI] [PubMed] [Google Scholar]
- 31. SAS Insititute, Inc. SAS Version 9.4 Cary, NC: SAS Institute, Inc.; 2013. [Google Scholar]
- 32. Gilbert ES. Some confounding factors in the study of mortality and occupational exposures. Am J Epidemiol. 1982;116(1):177–188. [DOI] [PubMed] [Google Scholar]
- 33. Costa LG, Cole TB, Coburn J, et al. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cole TB, Coburn J, Dao K, et al. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology. 2016;374:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Diesel and Gasoline Engine Exhausts and Some Nitroarenes. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 105). Lyon, France: International Agency for Research on Cancer; 2014. [PMC free article] [PubMed] [Google Scholar]
- 36. Eurostat Passenger cars in the EU. 2017. http://ec.europa.eu/eurostat/statistics-explained/index.php/Passenger_cars_in_the_EU. Accessed July 17, 2017.
- 37. Anenberg SC, Miller J, Minjares R, et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature. 2017;545(7655):467–471. [DOI] [PubMed] [Google Scholar]
- 38. US Environmental Protection Agency Diesel Engine Exhaust; CASRN N.A. (Integrated Risk Information System (IRIS) summary). Washington, DC: Environmental Protection Agency; 2003. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0642_summary.pdf. Accessed July 27, 2017. [Google Scholar]
- 39. Ilar A, Plato N, Lewné M, et al. Occupational exposure to diesel motor exhaust and risk of lung cancer by histological subtype: a population-based case-control study in Swedish men. Eur J Epidemiol. 2017;32(8):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silverman DT. Diesel exhaust causes lung cancer: now what? Occup Environ Med. 2017;74(4):233–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kurth L, Doney B, Weinmann S. Occupational exposures and chronic obstructive pulmonary disease (COPD): comparison of a COPD-specific job exposure matrix and expert-evaluated occupational exposures. Occup Environ Med. 2017;74(4):290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mills NL, Tornqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357(11):1075–1082. [DOI] [PubMed] [Google Scholar]
- 43. Barath S, Mills NL, Lundbäck M, et al. Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part Fibre Toxicol. 2010;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Calderón-Garcidueñas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32(6):650–658. [DOI] [PubMed] [Google Scholar]
- 45. Levesque S, Surace MJ, McDonald J, et al. Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malek AM, Barchowsky A, Bowser R, et al. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environ Pollut. 2015;197:181–186. [DOI] [PubMed] [Google Scholar]
- 47. Armon C. Accrued somatic mutations (nucleic acid changes) trigger ALS: 2005–2015 update. Muscle Nerve. 2016;53(6):842–849. [DOI] [PubMed] [Google Scholar]
- 48. Andrew AS, Caller TA, Tandan R, et al. Environmental and occupational exposures and amyotrophic lateral sclerosis in New England. Neurodegener Dis. 2017;17(2–3):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chió A, Meineri P, Tribolo A, et al. Risk factors in motor neuron disease: a case-control study. Neuroepidemiology. 1991;10(4):174–184. [DOI] [PubMed] [Google Scholar]
- 50. McGuire V, Longstreth WT Jr, Nelson LM, et al. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997;145(12):1076–1088. [DOI] [PubMed] [Google Scholar]
- 51. Weisskopf MG, McCullough ML, Morozova N, et al. Prospective study of occupation and amyotrophic lateral sclerosis mortality. Am J Epidemiol. 2005;162(12):1146–1152. [DOI] [PubMed] [Google Scholar]
- 52. Malek AM, Barchowsky A, Bowser R, et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neurodegener Dis. 2014;14(1):31–38. [DOI] [PubMed] [Google Scholar]
- 53. Gunnarsson LG, Lindberg G, Söderfeldt B, et al. Amyotrophic lateral sclerosis in Sweden in relation to occupation. Acta Neurol Scand. 1991;83(6):394–398. [DOI] [PubMed] [Google Scholar]
- 54. Bergman BP, Mackay DF, Pell JP. Motor neurone disease and military service: evidence from the Scottish Veterans Health Study. Occup Environ Med. 2015;72(12):877–879. [DOI] [PubMed] [Google Scholar]
- 55. Wang MD, Little J, Gomes J, et al. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology. 2017;61:101–130. [DOI] [PubMed] [Google Scholar]
- 56. Clemmensen KK, Lynge E, Clemmensen IH. Nationwide tobacco surveys and sales data in Denmark from 1920 to 2010. Dan Med J. 2012;59(6):A4448. [PubMed] [Google Scholar]
- 57. Hansen J, Raaschou-Nielsen O, Olsen JH. Increased risk of lung cancer among different types of professional drivers in Denmark. Occup Environ Med. 1998;55(2):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Osler M. Smoking habits in Denmark from 1953 to 1991: a comparative analysis of results from three nationwide health surveys among adult Danes in 1953–1954, 1986–1987 and 1990–1991. Int J Epidemiol. 1992;21(5):862–871. [DOI] [PubMed] [Google Scholar]
- 59. Sutedja NA, Veldink JH, Fischer K, et al. Lifetime occupation, education, smoking, and risk of ALS. Neurology. 2007;69(15):1508–1514. [DOI] [PubMed] [Google Scholar]
- 60. de Jong SW, Huisman MH, Sutedja NA, et al. Smoking, alcohol consumption, and the risk of amyotrophic lateral sclerosis: a population-based study. Am J Epidemiol. 2012;176(3):233–239. [DOI] [PubMed] [Google Scholar]
- 61. Wang H, O’Reilly EJ, Weisskopf MG, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68(2):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gallo V, Bueno-De-Mesquita HB, Vermeulen R, et al. Smoking and risk for amyotrophic lateral sclerosis: analysis of the EPIC cohort. Ann Neurol. 2009;65(4):378–385. [DOI] [PubMed] [Google Scholar]
- 63. Weisskopf MG, McCullough ML, Calle EE, et al. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol. 2004;160(1):26–33. [DOI] [PubMed] [Google Scholar]
- 64. Alonso A, Logroscino G, Hernán MA. Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2010;81(11):1249–1252. [DOI] [PubMed] [Google Scholar]
- 65. Osler M, Holstein B, Avlund K, et al. Socioeconomic position and smoking behaviour in Danish adults. Scand J Public Health. 2001;29(1):32–39. [PubMed] [Google Scholar]
- 66. Kioumourtzoglou MA, Seals RM, Himmerslev L, et al. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3-4):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Coppedè F. An overview of DNA repair in amyotrophic lateral sclerosis. ScientificWorldJournal. 2011;11:1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.