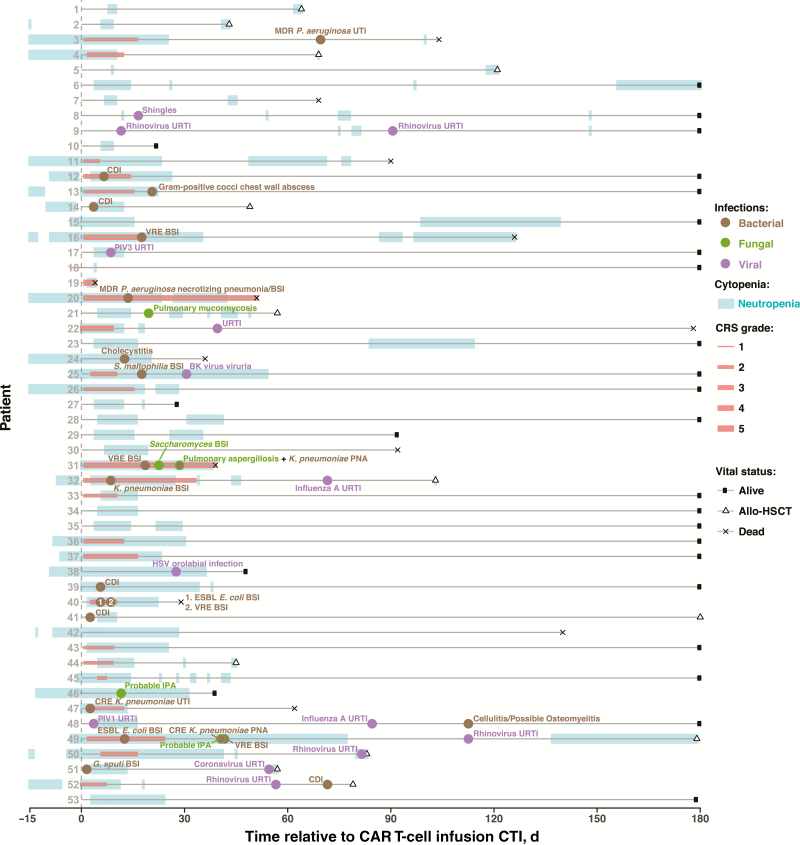
Figure 1.
Overview of infections and clinical events in patients treated with chimeric antigen receptor (CAR) T cells. Timeline depicts the periods of neutropenia, cytokine release syndrome, and bacterial, fungal, and viral infections for 53 study patients after CAR T-cell infusion (CTI) until allogeneic transplantation, death, or last follow-up date through day 180. Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; BSI, bloodstream infection; CDI, Clostridium difficile infection; CRE, carbapenem-resistant Enterobacteriaceae; CTI, CAR T-cell infusion; E. coli, Escherichia coli; ESBL, extended-spectrum β-lactamase; G. sputi, Gordonia sputi; HSV, herpes simplex virus; IPA, invasive pulmonary aspergillosis; K. pneumoniae, Klebsiella pneumoniae; MDR, multidrug-resistant; P. aeruginosa, Pseudomonas aeruginosa; PIV, parainfluenza virus; PNA, pneumonia; S. maltophilia, Stenotrophomonas maltophilia; URTI, upper respiratory tract infection; UTI, urinary tract infection; VRE, vancomycin-resistant Enterococcus.