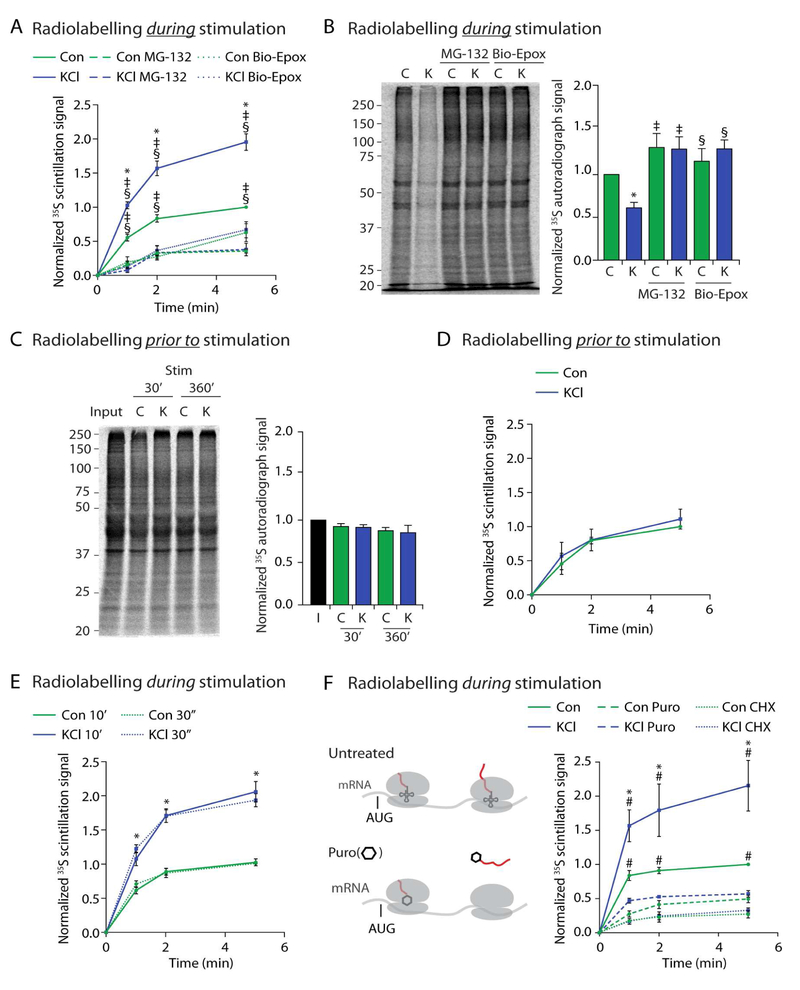
Figure 1. Neuronal stimulation induces NMP-dependent degradation of newly synthesized proteins into extracellular peptides.
(A) Concomitant radiolabelling during neuronal stimulation induces NMP-mediated peptide release. Scintillation data at the indicated time points are shown normalized to control at the 5-minute time point. Data are presented as mean ± SEM (n = 3). *p < 0.01 (KCl), ‡p < 0.01(MG132), §p < 0.01 (Bio-Epox)(two-way ANOVA).
(B) Neuronal stimulation induces NMP-mediated degradation of intracellular proteins made during stimulation. Left, representative autoradiograph of lysates from neurons radiolabelled during either control (C) or KCl (K) stimulation and treated with MG132 or biotin-epoxomicin (Bio-Epox). Right, quantification of densitometry signal normalized to control. Data are presented as mean ± SEM (n = 3). *p < 0.01 (Control), ‡p < 0.01 (MG132), §p < 0.01 (Bio-Epox)(two-way ANOVA).
(C) Neuronal stimulation does not induce NMP-mediated degradation of proteins made prior to stimulation. Left, Representative autoradiograph of lysates from neurons previously radiolabelled and then chased into either control (C) or KCl (K) stimulation buffers for indicated times. Input shows sample collected immediately following labeling. Right, quantification of densitometry signal normalized to control. Data are presented as mean ± SEM (n = 3). Statistically significant differences between samples was not observed (two-way ANOVA).
(D) Neuronal stimulation does not induce NMP-mediated degradation of proteins made prior to stimulation. Experiments done as described in (A), note neurons were radiolabelled prior to instead of during stimulation as in (A). Data are presented as mean ± SEM (n = 3). Statistically significant differences between samples was not observed (two-way ANOVA).
(E) Peptide release following 30 second (dotted lines) or 10 minutes (solid lines) of radiolabeling as in (A). Data are presented as mean ± SEM (n = 3). Line graph, *p < 0.01 (two-way ANOVA) for Control compared to KCl for either 30 second or 10 minute labeling. No significance was detected between similar treatments at 30 seconds versus 10 minutes.
(F) Concomitant radiolabelling during neuronal stimulation induces NMP-mediated radiolabeled peptide release that is sensitive to cycloheximide and puromycin treatment. (Left) Translating ribosomes (grey) on mRNA. AUG start site shown just prior to tRNA (small structure with codon recognition loops, in ribosome P site) and growing radioactive polypeptide (growing red line out of translating ribosomes). Puromycin (hexagon) modifies and releases the nascent polypeptide (red) from actively translating ribosomes. Experiments done as described in (A), note, washout with fresh media containing Cycloheximide (CHX) or puromycin (Puro). Data are presented as mean ± SEM (n = 3). *p < 0.01 (Control), #p < 0.01 (puromycin) (two-way ANOVA).
See also Figure S1.