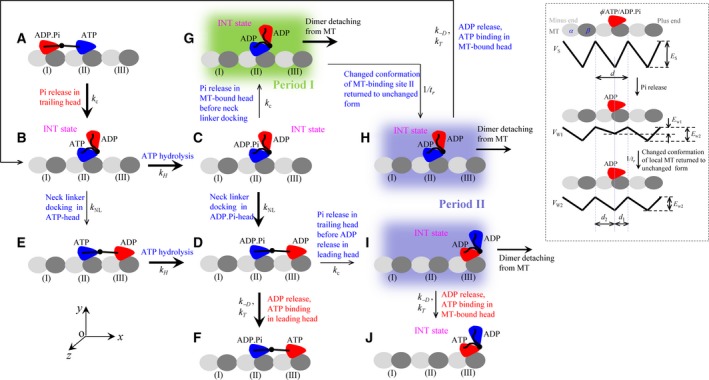
Schematic illustrations of the model of a typical forward stepping of the dimer at saturating ATP. Inset shows interaction potentials between the single kinesin head and MT along a MT protofilament during an ATPase cycle, with the top panel showing the strong interaction potential, VS, in nucleotide‐free (φ), ATP, or ADP.Pi states; the middle panel showing the weak interaction potential, VW
1, in ADP state immediately after Pi release; and the bottom panel showing the weak interaction potential, VW
2, in ADP state in a period of time t
r after Pi release. (A) The trailing head in ADP.Pi state binds strongly to the rear MT‐binding site (I) while the leading head in ATP state binds strongly to the forward MT‐binding site (II). The trailing head with its NL pointing forward has a larger Pi release rate than the leading head with its NL not pointing forward. (B) Upon Pi release in the trailing head, due to the very weak affinity (E
w1) between the ADP–head and the local MT‐binding site (I), driven by the thermal noise, the trailing ADP–head diffuses rapidly to the intermediate position relative to the other MT‐bound head, where the two heads have strong affinity. (C) ATP is hydrolyzed to ADP.Pi in the MT‐bound head. (D) In the intermediate state with the MT‐bound head in ADP.Pi state, the NL docking takes place, weakening the interaction between the two heads. Then, the thermal noise drives the tethered head to diffuse rapidly to the forward MT‐binding site (III). (E) In the intermediate state of (B), before ATP is hydrolyzed to ADP.Pi in the MT‐bound head, NL docking can also take place with a very low probability due to the very slow rate of NL docking in ATP state, weakening the interaction between the two heads. The tethered head then diffuses rapidly to the forward MT‐binding site (III). (F) Stimulated by MT ADP is released rapidly, followed by ATP binding. (G) From (C), Pi release can also occur occasionally in the MT‐bound head before its NL docking. Before the affinity of the MT‐bound ADP–head for the local MT‐binding site (II) changes from E
w1 to E
w2, the dimer can easily detach from MT by overcoming the very weak affinity E
w1. (H) From (G) the affinity of the MT‐bound ADP–head for the local MT‐binding site (II) changes from E
w1 to E
w2 in time t
r. The dimer also has a large probability to detach from MT before ADP release from the MT‐bound head by overcoming weak affinity E
w2. If the dimer has not detached until ADP release, which is followed by ATP binding, the system returns to (B). (I) From (D), Pi release can also occur occasionally in the trailing head before ADP release in the leading head. The dimer has a large probability to detach from MT before ADP release from the MT‐bound head by overcoming weak affinity E
w2. (J) From (I), the dimer has not detached until ADP release, which is followed by ATP binding. The thickness of the arrow represents the magnitude of the transition probability under no load. The states (shaded in green and blue) where the dimer can detach from MT are indicated.