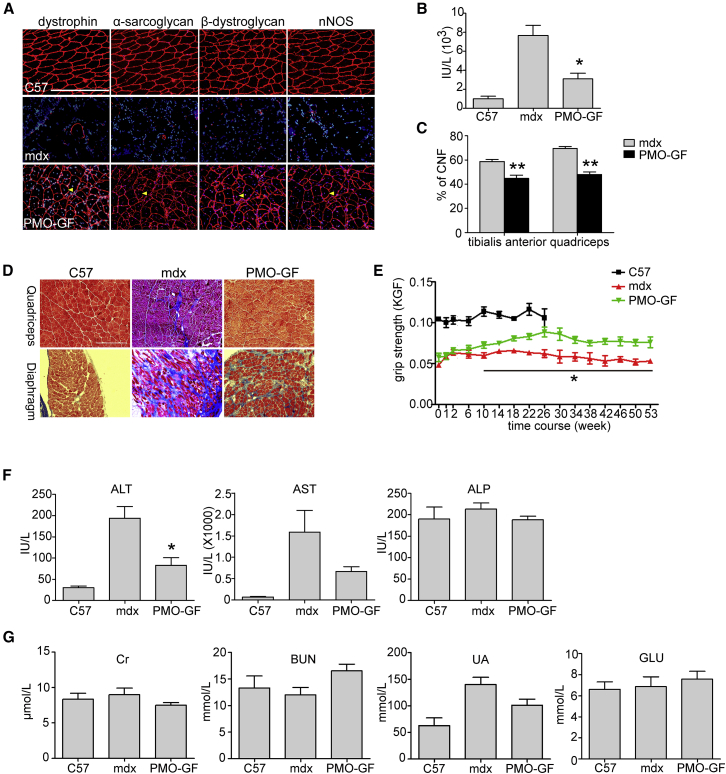
Figure 2.
Functional Improvement in mdx Mice Treated with PMO-GF for 1 Year
Functional improvement in mdx mice treated with PMO-GF at the dosage of 50 mg/kg/week for 3 weeks followed by 50 mg/kg/month for 11 months intravenously. (A) Re-localization of dystrophin-associated protein complex (DAPC) components in treated mdx mice to assess dystrophin function and recovery of normal myoarchitecture. (B) Measurement of serum creatine kinase (CK) levels. Data show a significant fall in mdx mice treated with PMO-GF compared with untreated age-matched mdx controls (two-tailed t test, n = 5, *p < 0.05). (C) Evaluation of CNFs in tibialis anterior and quadriceps from mdx mice treated with PMO-GF. A significant decrease was detected between PMO-GF-treated mdx mice and age-matched mdx controls (two-tailed t test, n = 5, **p < 0.01). (D) Collagen deposition analysis in diaphragm and quadriceps from mdx mice treated with PMO-GF (scale bar, 200 μm). (E) Muscle function was assessed to determine the physical improvement. A significant improvement was detected between PMO-GF-treated mdx mice and untreated age-matched mdx controls at different time points (two-tailed t test, n = 5, *p < 0.05). (F) Measurement of serum levels of liver enzymes in mdx mice treated with PMO-GF compared with untreated mdx controls. Data show improved pathological parameters in mdx mice treated with PMO-GF compared with untreated age-matched mdx controls (two-tailed t test, n = 5, *p < 0.05). (G) Analysis of biochemical indicators for kidney function and glucose in mdx mice treated with PMO-GF. Data show no difference in the level of serum creatinine (Cr), BUN, urea, and glucose in mdx mice treated with PMO-GF compared with untreated mdx and normal controls (n = 5).