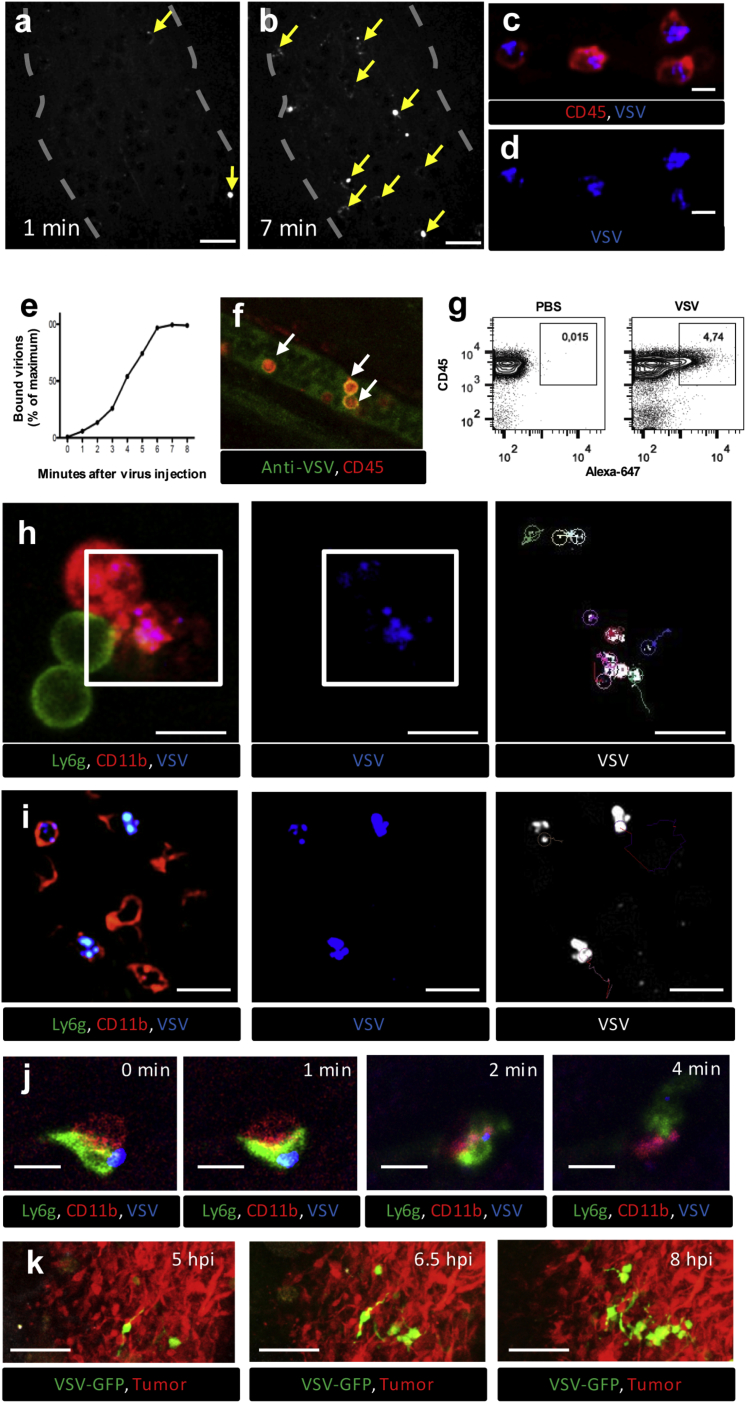
Figure 4.
IVM Imaging of OV-Host Interactions in Mouse Tumors
(A and B) 5 × 108 PFU VSV labeled with 30 μg/mL AF647 were injected i.v. while visualizing superficial CT-26 tumor vessels by confocal IVM. Arrows indicate virus particles (white) bound to leukocytes (shadows) at 1 (A) and 7 (B) min following virus injection (see also Video S3). Scale bars, 20 μm; representative images of three independent experiments. (C and D) IVM of CD45 cells (red) bound by VSV-AF647 (blue) in the CT-26 tumor vessels (see also Video S4) (C, merged channels; D, AF647 channel only). Scale bars, 10 μm; representative images of three independent experiments. (E) Dynamics of virus binding by CD45+ cells inside the CT-26 tumor vessels during first 8 min after i.v. injection. Values represent the percentage of maximum virus binding by CD45+ cells within the field of view (representative plot of three independent experiments). (F) Confirmation of VSV binding to CD45 leukocytes using VSV antibody. CT-26-bearing mice were treated i.v. with unlabeled VSV (5 × 108 PFU) and, 5 min later, with antibody targeting VSV (green) and CD45 (red). Tumor vessels were imaged using confocal IVM. White arrows indicate VSV-bound CD45 leukocytes. Scale bars, 20 μm; representative image of two independent experiments. (G) Flow cytometry analysis of blood collected 5 min after i.v. injection of PBS (left panel) or AF647-labeled VSV (right panel) (representative plot of three independent experiments). (H) Tracking of individual viral particles 5 min after i.v. injection of AF647-labeled VSV. Time-lapse imaging was collected for 120 s, and individual virus particle trajectories on the surface of CD11b+/Ly6g− cells in vessels were reconstructed using ImageJ (see also Video S5) (left, merged channels [red, CD11b; green, Ly6g; blue, VSV]; middle, AF647 channel only; right [selected area from left and middle], viral trajectories). Scale bars, 10 μm; representative images of three independent experiments. (I) Tracking of intravascular leukocytes 5 min after i.v. injection of AF647-labeled VSV. Time-lapse imaging was collected for 200 s, and the trajectories of intravascular leukocytes bound by virus were reconstructed using ImageJ (see also Video S6) (left, merged channels [red, CD11b; green, Ly6g; blue, virus]; middle, AF647 channel only; right, cell trajectories). Scale bars, 20 μm; representative images of three independent experiments. (J) Tracking of individual viral particles 30 min after i.v. injection of AF647-labeled VSV. Time-lapse imaging of virus (blue) transfer from monocyte (red, CD11b) to neutrophil (green, Ly6g) within the blood vessel (gray) 30 min after i.v. injection of AF647-labeled VSV (see also Video S7). Scale bars, 10 μm; representative images of three independent experiments. (K) IVM of VSV spreading through CT-26 tumors. CT-26RFP-bearing mice were treated i.v. with VSVGFP (5 × 108 PFU). Growing foci of VSV infection were imaged by IVM between 5 and 8 hpi. Green, VSV; red, CT-26 tumor cells; scale bars, 100 μm; representative images of three independent experiments.