Abstract
Transcription factor RUNX1 holds an integral role in multiple-lineage haematopoiesis and is implicated as a cofactor in V(D)J rearrangements during lymphocyte development. Runx1 deficiencies resulted in immaturity and reduction of lymphocytes in mice. In this study, we found that runx1W84X/W84X mutation led to the reduction and disordering of B cells, as well as the failure of V(D)J rearrangements in B cells but not T cells, resulting in antibody-inadequate-mediated immunodeficiency in adult zebrafish. By contrast, T cell development was not affected. The decreased number of B cells mainly results from excessive apoptosis in immature B cells. Disrupted B cell development results in runx1W84X/W84X mutants displaying a similar phenotype to common variable immunodeficiency—a primary immunodeficiency disease primarily characterized by frequent susceptibility to infection and deficient immune response, with marked reduction of antibody production of IgG, IgA and/or IgM. Our studies demonstrated an evolutionarily conserved function of runx1 in maturation and differentiation of B cells in adult zebrafish, which will serve as a valuable model for the study of immune deficiency diseases and their treatments.
Keywords: Runx1 mutation, lymphocyte, immunodeficiency, zebrafish
1. Introduction
Multipotent haematopoietic stem cells germinate lymphoid-restricted progenitors that differentiate into subsets of B and T cells. Lymphocytes play a vital role in adaptive immunity, while B cells provide varied immunoglobulin antibodies which perform the humoral immune response [1]. The matured B cells can differentiate into plasmocytes and memory cells to eliminate pathogens and maintain the health of individuals via the immune response. Therefore, the dysregulation of the production and functionality of B cells often results in a variety of human diseases, including leukaemia [2], common variable immunodeficiency (CVID) [3] and X-linked agammaglobulinaemia [4]. CVID refers to a heterogeneous collection of primary immunodeficiency diseases, primarily characterized by frequent susceptibility to infection and deficient immune response, concomitant with a marked reduction in antibody production of IgG, IgA and/or IgM. Defective lymphocyte development, especially of B cells, is the major pathophysiological cause of CVID [5].
Mammalian B cell development is a sequential process, which can be divided into seven stages: pre-pro-B cell, pro-B cell, pre-B cell, immature B cell, mature B cell, activated B cell and plasma B cell [6,7]. V(D)J rearrangements of B cells are responsible for producing the Ig heavy chains during the B cell maturation. Complex pathways and multiple genes drive B lymphopoiesis, including B cell maturation, commitment, specification and differentiation. Mouse and cell line studies have shown that Pax5 (B cell-specific activator protein, BSAP) has concerted action with a set of genes such as Runx1, Blk, E2a, Ikaros and other B-lineage-specific transcription factors to establish the B cells development network [6,8–10]. Coordination failure between different signalling molecules and regulators would result in defects in B lymphopoiesis. RUNX1 is a critical member of the RUNX (runt-related) family harbouring highly conserved DNA binding and protein–protein interaction domains as heterodimeric transcription factors in vertebrate [11–14]. Mutations in Runx1 are known to be strongly and frequently associated with haematological malignancies [15], where RUNX1 serves as a key regulator in the initiation and maintaining a steady state of haematopoietic stem cell [16–18], the emergence of thrombocytes [19] and growth of lymphocytes [20–22]. A recent survey study concluded that of 128 patients with acute lymphoblastic leukaemia, approximately 18.3% patients with T cell acute lymphoblastic leukaemia and 3.8% patients with B cell acute lymphoblastic leukaemia carried a RUNX1 mutation [23]. Several genes have been shown to be the downstream targets of Runx1, including Ebf1, Ly6d, Spib and Ikzf3 [20,21]. However, the mechanism and signalling pathway of RUNX1 in modulating lymphocyte development remain incompletely elucidated.
Haematopoietic processes of zebrafish are evolutionarily similar to mammalian processes, including lymphopoiesis [24]. Zebrafish has been an excellent vertebrate genetic and developmental system for disease analysis, contributing a valuable increase in the understanding of haematopoiesis and the immune system [25,26]. In zebrafish, the thymus is generally the first to develop as a lymphoid organ accumulating T cells [27], which is initiated by expression of rag1/2. B cells emerge from 21 days post-fertilization (dpf) in the pronephros and kidney marrow [28], in which large antibody repertoires exist [29]. The conserved haematopoietic programme of zebrafish has served as a versatile model organism to demonstrate the events in vertebrate lymphoid ontogeny.
The tightly regulated network of runx1 activation has been studied extensively in humans and mice, but not in adult zebrafish. We have established a CVID model by utilizing zebrafish runx1W84X/W84X mutants, which mimic the haematopoietic and immunodeficiency of B cells. Using these mutants, we address how runx1 regulates B cell growth and the mechanism of CVID. Our results indicated a dramatic decrease of B cell numbers, ineffective immune response and aborted V(D)J rearrangements of B cells in runx1W84X/W84X mutants, demonstrating a conserved role of runx1 in B cell development. This model can be used for exploring potential therapies for CVID.
2. Results
2.1. Abnormal development of lymphocytes in runx1W84X/W84X mutants
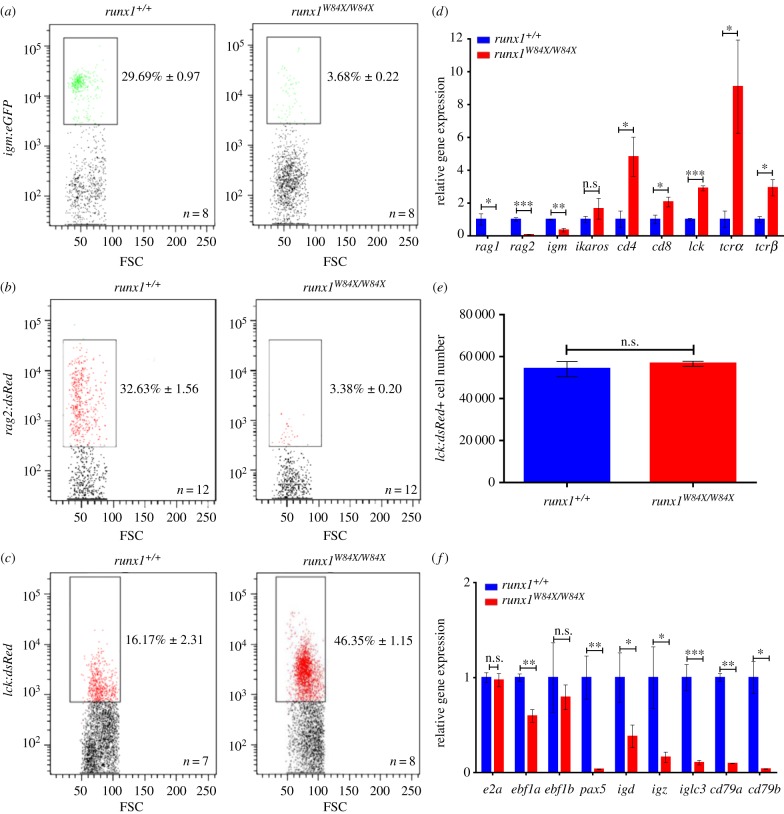
Runx1 is essential for survival and for the continued development of B cells and T cells in mice [20,21,30,31]. To gain insights into the roles of runx1 in adult zebrafish lymphocyte development, we used runx1W84X/W84X mutants that produce truncated proteins and lack runx1 function [32,33]. As in other teleosts, adult zebrafish maintain multi-lineage haematopoiesis in the kidney marrow, an organ that is equivalent to the mammalian bone marrow, the source of B cells and T cells. We used Tg(igm:eGFP), Tg(rag2:dsRed) and Tg(lck:dsRed) transgenic lines; these closely recapitulate mammalian B cell [34,35] and T cell ontogeny, respectively. FACs analysis demonstrated that runx1W84X/W84X mutants showed sharply reduced percentages of igm:eGFP+ and rag2:dsRed+ B cells but expanded lck:dsRed+ T cells compared with runx1+/+ (figure 1a–c). We then examined the expression of B-cell- and T-cell-related genes in lymphocytic populations of kidney marrow from adult runx1+/+ and runx1W84X/W84X mutants using Q-RT-PCR [34]. Specific primers were designed as seen in table 1. As expected, we found decreased B cell genes and increased T cell genes (figure 1d), indicating that T cell number may not be affected or may even increase. To more accurately discern whether runx1 would affect T cell development, we measured T cell number in the kidney (figure 1e). The absolute T cell number in runx1W84X/W84X mutants is comparable to that of runx1+/+. Therefore, the increase in T cell percentage is likely to be the mathematical consequence of the reduction in B cell percentage, rather than the increase of T cell quantity.
Figure 1.
Aberrant developmental characterization of lymphocytes in adult runx1W84X/W84X mutants. (a–c) FACS analysis of B cell makers igm (a) and rag2 (b), T cell maker lck (c) in kidney marrow of runx1+/+ and runx1W84X/W84X mutants. Black boxes outline the captured positive lymphocytes with fluorescence in lymphocytic populations of kidney marrow; the percentage data represents the mean ± s.e.m. (d) Relative expression of B cell makers and T cell makers in runx1+/+ (blue bars, n = 54) and runx1W84X/W84X mutants (red bars, n = 54) were examined by Q-RT-PCR. (e) Absolute numbers of T cells in runx1+/+ (blue bar, n = 3)and runx1W84X/W84X mutants (red bar, n = 3). (f) Relative expression of genes important at multiple stages of B cells development. Each experiment was performed in duplicate. Unpaired Student's t-test; ns, no significance; *p < 0.05; **p < 0.01; ***p < 0.001.
Table 1.
Primers used in Q-RT-PCR.
| gene | FP | RP |
|---|---|---|
| ef-1α | TACTTCTCAGGCTGACTGTG | ATCTTCTTGATGTATGCGCT |
| rag1 | AATGATGCAAGGCAGAGGA | CAATGATGCCCACATCCC |
| rag2 | TGAGACTCAGAAGCGCATGG | ACCAAGTACGACTGTGGCTG |
| igm | GTTTCCTCAGCTCAACCA | AGTATAATCTCCTTCCTTCCC |
| ikaros | AGAAGG GTAACCTGCTCCGACAC | GGGCTT TCCAACCGAATGAGT |
| cd4 | GTGGTCTTCATCTTGCTTGT | AATCCCTTTGGCTGTTTGTT |
| cd8 | AAGAGCATAGCACCGTAG | GACTTCCGTCTGCTTTGCG |
| lck | ACGTAAACATGGGGAACTG | TCTTCTCCCCTTTCTCAAAC |
| tcrα | GAAGCCGAATATTTACCAAGTG | AACAAACGCCTGTCTCCT |
| tcrβ | AAATCAACAAACAAATTCACCTG | TATGCCAGCTTCATCCACTG |
| e2a | AATGTGCAAGAAGGACTTCCAGATC | CATGATGCCTTCGCTGGAGCTGAT |
| ebf1α | TTTACAGCAACGGCATCAGAACAG | GGTTACATTTGAGGAAGAATTTCAGG |
| ebf1β | ATCATATTTGGAGCATGCTGCACCT | CTTATAGGAGAGTGTGACCTCTACC |
| pax5 | CTGATTACAAACGCCAAAAC | CTAAATTATGCGCAGAACG |
| igd | GACACATTAGCCCATCAGCA | CTGGAGAGCAGCAAAAGGAT |
| igz | AAAGCAACGATACCAAAGTG | AACAGCTTGCAAGACAATTC |
| iglc3 | AAGGAACTAAACCCATTGTGACGGA | TCGCTGCATTCAGATTTCCTGATG |
| cd79a | TCAAGAATACTCCCGCCATC | GGCTTCTCCAGCTGAATGTC |
| cd79b | GCTCACTTACGAATGACCAGAGAATAAC | GTCCTCATACACATCTCCACCAACC |
| distal primer | ACTAGATGACAATGTTGCGCTGGCAAC | CAGTTGGGGGTAATTATGACTAACAAAAGTGCT |
| proximal primer | GACAGCTAATGGTAGTTCGGCTTACTTATG | CTTGTGGAGACAGCTCCCTCGCTGTTC |
Additionally, B cells from another immune organ, spleen, were reduced in runx1W84X/W84X mutants (electronic supplementary material, figure S1A,B). To determine the precise developmental stages during which B cell differentiation is blocked, we analysed the expression of B-cell-specific genes such as e2a, ebf1α, ebf1b, pax5, igd, igz, iglc3, cd79a and cd79b which are important at multiple stages of B cell development [36–38] (figure 1f). These results indicated that B cells had been deficient from an early stage of B cell development in runx1W84X/W84X mutants, similar to the Runx1 knock-out mouse [20].
2.2. B cells but not T cells are dysfunctional in runx1W84X/W84X mutants: establishment of common variable immunodeficiency model
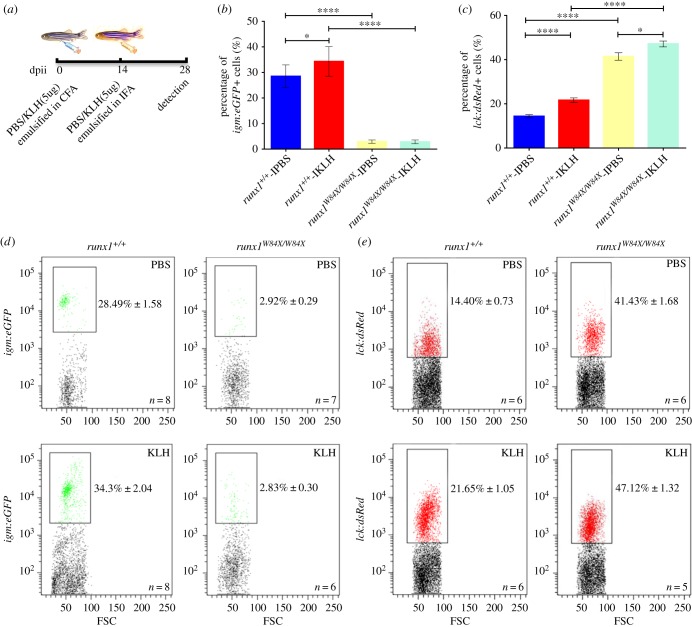
We observed that although runx1W84X/W84X mutants were similar in size to runx1+/+, 35 of 687 (about 5.09%) runx1W84X/W84X mutants appeared frail and ill, suffering recurrent infection and displaying anomalies in sustained swimming and morphology (electronic supplementary material, figure S2A). By recording the gross survival rate of runx1+/+ and runx1W84X/W84X mutants every day, we found a much lower survival rate of runx1W84X/W84X mutants compared with runx1+/+ (electronic supplementary material, figure S2B). The dysplasia of B cells and T cells prompted us to test the immune function of runx1W84X/W84X mutants by measuring the immune response [35,39]. To this end, phosphate buffer solution (PBS) as the control and KLH emulsified in complete Freund's adjuvant were used as antigen to immune Tg(igm:eGFP);runx1+/+ and Tg(igm:eGFP);runx1W84X/W84X mutants, Tg(lck:dsRed);runx+/+ and Tg(lck:dsRed);runx1W84X/W84X mutants. Two weeks later, PBS and KLH emulsified in incomplete Freund's adjuvant (IFA) boosted the immune fish (figure 2a). After four weeks, the percentage of igm:eGFP+ B cells in lymphocytes were increased in Tg(igm:eGFP);runx1+/+, but not in Tg(igm:eGFP);runx1W84X/W84X mutants (figure 2b,d). The data indicated that the residual B cells in runx1W84X/W84X mutants lacked immune response to antigen stimulation. In contrast, compared with Tg(lck:dsRed);runx+/+, the immune response of lck:dsRed+ T cells in Tg(lck:dsRed);runx1W84X/W84X mutants was normal (figure 2c,e). These data provided the conclusion that loss of runx1 function in zebrafish resulted in dysfunctional development of B cells but not that of T cells, a phenotype resembling CVID with reduced B cells, defects in B cell development, and impaired secretion of immunoglobulin in humans [40].
Figure 2.
Deficient immune function of B cells but not T cells in adult runx1+/+ and runx1W84X/W84X mutants kidney marrow. (a) Scheme of immune responses procedure showing dose, assigned groups and process involved in intraperitoneal injection of KLH. Percentage of igm:eGFP+ and lck:dsRed+ cells in lymphocytes of kidney marrow were detected at 28dpii. dpii, day post intraperitoneal injection; PBS, phosphate buffer solution; KLH, keyhole limpet haemocyanin; CFA, complete Freund's adjuvant; IFA, incomplete Freund's adjuvant. (b–e) Quantified percentage and FACS analysis of igm:eGFP+ cells (b,d) and lck:dsRed+ cells (c,e) in lymphocytes of kidney marrow from assigned groups at 28dpii. Each experiment was performed in duplicate. Blue bars indicate the runx1+/+-IPBS, red bars indicate the runx1+/+-IKLH, yellow bars indicate the runx1W84X/W84X-IPBS, light blue bars indicate the runx1W84X/W84X-IKLH; black boxes outline the captured positive lymphocytes with fluorescence in lymphocytic population of kidney marrow; ANOVA, Dunnett T3; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
2.3. Apoptosis of B cells is increased in runx1W84X/W84X mutants
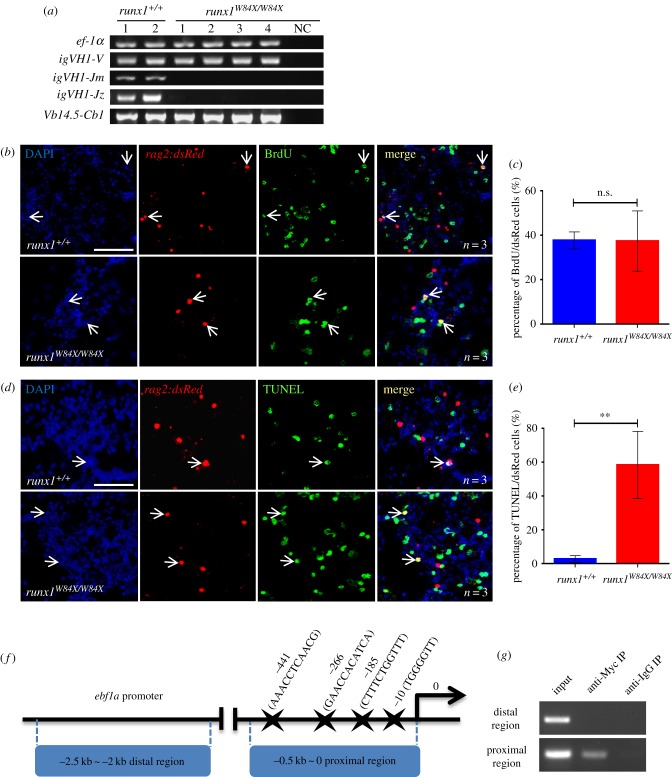
It is known that lymphocytic V, D and J gene segment rearrangements are the initial essential events that occur in the maturation of B cells and T cells. Adult runx1W84X/W84X mutants have abnormal development of B cells and/or T cells. We intended to detect V(D)J rearrangements of B cells and T cells in kidney from runx1+/+ and runx1W84X/W84X mutants by semi-nested PCR assays. The expression of Ig heavy chain isotypes igm and igz, in addition to T cell receptor tcrβ rearrangements, were checked by the primers listed in table 2 [41–43]. Igm, igz and tcrβ were robustly amplified in both runx1+/+ and runx1W84X/W84X mutants. runx1W84X/W84X mutants had nearly undetectable igm and igz rearrangement expression but had normal tcrβ transcriptional rearrangement expression in the kidney (figure 3a). These results imply that, similar to the Runx1-null mutation in mice, B cell rearrangements were interrupted in runx1W84X/W84X mutants. This suggests a functional requirement of runx1 in the maturation and/or maintenance of B cells in zebrafish. Moreover, we found that in zebrafish, runx1's role is indispensable in the development of B cell but not T cells.
Table 2.
Primers used in rearrangement assays.
| gene | FP | RP |
|---|---|---|
| ef-1α | TATCTCCAAGAACGGACAGAC | GCAAACTTGCAGGCGATGTG |
| igVH1-V (first round) | GATGGACGTGTTACAATTTGG | CGTGCACAGTAATAAACAGCT |
| igVH1-V (second round) | CCTCCTCAGACTCTGTGGTGA | CGTGCACAGTAATAAACAGCT |
| igVH1-Jm (first round) | GATGGACGTGTTACAATTTGG | GTTCCYTTHCCCCAGTAGTCAAA |
| igVH1-Jm(second round) | CCTCCTCAGACTCTGTGGTGA | GTTCCYTTHCCCCAGTAGTCAAA |
| igVH1-Jz (first round) | GATGGACGTGTTACAATTTGG | AAGGTCTATTACTAACAGATCAC |
| igVH1-Jz(second round) | CCTCCTCAGACTCTGTGGTGA | AAGGTCTATTACTAACAGATCAC |
| Vb14.5-Cb1(first round) | GAATCCAATGTGACGTTAACATGC | AAGATGACAAGGCCATACAGTC |
| Vb14.5-Cb1(second round) | CATGATCATAAGGACCACTACAG | GTCCGCTCTTAGCAATGGTC |
Figure 3.
Deficiency of B cells development in runx1W84X/W84X mutants. (a) V(D)J rearrangements of igm, igz and V(DJ)C rearrangement of tcrβ analysis in kidney marrow from runx1+/+ (n = 2) and runx1W84X/W84X mutants (n = 4) by semi-nested PCR. The ef-1α and igVH1-V PCR were used as positive control. NC, negative control. (b–c) Cell proliferation assay of B cells in kidney marrow of runx1+/+ and runx1W84X/W84X mutants. (b) Triple staining of DAPI, rag2:dsRed and BrdU. Blue: DAPI, red: dsRed, green: BrdU; arrows indicate DAPI staining, rag2:dsRed staining, BrdU staining and triple co-staining cells. (c) Comparison of percentage of rag2:dsRed+ cells for BrdU co-staining cells between runx1+/+ (blue bar, n = 3) and runx1W84X/W84X mutants (red bar, n = 3). Unpaired Student's t-test; p < 0.05; ns, no significance; mean ± s.e.m. (d–e) Cell apoptosis assay of B cells in kidney marrow of runx1+/+ and runx1W84X/W84X mutants. (d) Triple staining of DAPI, rag2:dsRed and TUNEL. Blue: DAPI, red: dsRed, green: TUNEL; arrows indicate DAPI staining, rag2:dsRed staining, TUNEL staining, and triple co-staining cells. (e) Comparison of percentage of rag2:dsRed+ cells for TUNEL between runx1+/+ (blue bar, n = 3) and runx1W84X/W84X mutants (red bar, n = 3). (f) Schematic diagram of the 2.5 kb ebf1a promoter region. The transcription initiation site is designated as 0. Putative Runx1 consensus sites (marked by stars) are shown. (g) Semi-quantitative PCR analysis of the enrichment of the −0.5 ∼ 0 kb proximal region (ii) and the −2.5 ∼ −2 kb distal region (i). The left lanes were input DNA control. Each experiment was performed in duplicate. Unpaired Student's t-test; mean ± s.e.m; **p < 0.01; scales bars, 100 µm.
In theory, reduced B cells in kidney from runx1W84X/W84X mutants could be due to any one or more of the three following underlying cellular abnormalities: (1) impaired proliferation of existing B cells; (2) reduced de novo B cells; (3) enhanced apoptosis of B cells. In the light of the essential role of runx1 in proliferation and cell apoptosis, we next monitored the proliferation and cell apoptosis of B cells via BrdU incorporation assay and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labelling) assay [44] respectively to survey which cellular mechanisms mediated the reduced B cells in runx1W84X/W84X mutants. Quantification of the DAPI/rag2:dsRed/BrdU triple positive cells population showed the B cell proliferation was similar between runx1+/+ and runx1W84X/W84X mutants (figure 3b,c). However, measurement of the DAPI/rag2:dsRed/TUNEL triple positive cell population showed a significantly increased ratio in runx1W84X/W84X mutants compared with runx1+/+, suggesting that B lymphocyte apoptosis was increased (figure 3d,e).
It was reported that Runx1 controls B lymphopoiesis by regulating Ebf1 [20], and loss of Ebf1 results in increased apoptosis in pro-B cells [45]. Many EBF1 target genes, for example, components of the pre-BCR and BCR, Cd79a, Cd79b, B lymphoid kinase, Vpreb1, Igll1 and Cd19 genes, are required for B cell survival [46]. In this study, we found that runx1W84X/W84X mutation led to the reduction of ebf1, pax5, rag1/2, cd79a and cd79b, which are necessary for B cell survival (figure 1d,f). In addition, we detected the binding of Runx1 to the zebrafish ebf1a promoter. Four putative Runx1 consensus sites identified within −0.5 ∼ 0 kb proximal region using JASPAR online software, with their positions (marked by stars) relative to the transcription initiation site, are shown (figure 3f). Chromatin immunoprecipitation (ChIP) showed that Myc-tagged Runx1 binds to the −0.5 ∼ 0 kb proximal but not distal promoter region (−2.5 ∼ −2 kb) of the ebf1a promoter (figure 3g). These Runx-binding motifs may be essential for gene expression, suggesting that Runx1 may directly control the transcription of ebf1a. Therefore, runx1 is likely to promote B cell development by regulating key factors such as ebf1a, which in turn regulates pax5, its known downstream target, to control the development of B cells.
2.4. Runx1 regulates B cells development in a cell-autonomous manner in zebrafish
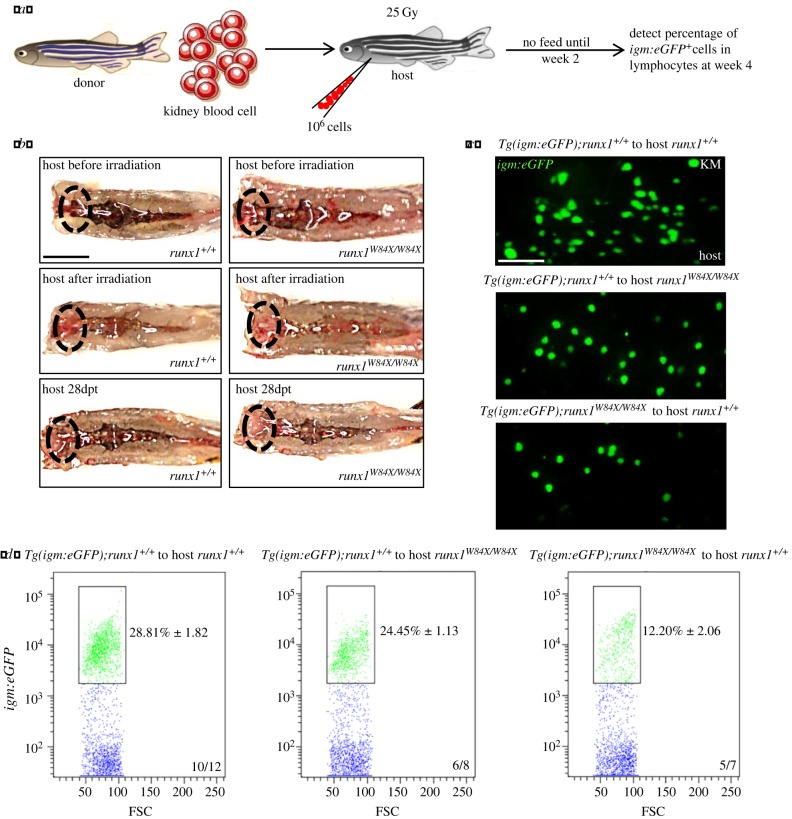
The importance of the expression of runx1 in haematopoiesis raised the question of whether runx1 is required cell-autonomously or non-cell-autonomously for the development of B cells in runx1W84X/W84X mutant zebrafish. To determine between the two possibilities, we conducted reciprocal kidney transplantation experiments between runx1+/+ and runx1W84X/W84X mutants [47–49] (figure 4a). We transplanted whole kidney blood cells from Tg(igm:eGFP);runx1+/+ donor to runx1+/+ host, Tg(igm:eGFP);runx1+/+ donor to runx1W84X/W84X mutant host and Tg(igm:eGFP);runx1W84X/W84X mutant donor to runx1+/+ host. We found that in Tg(igm:eGFP);runx1+/+ to runx1+/+ group, 10 of 12 hosts have runx1+/+-phenotyped B cell reconstitution (figure 4b–d); in Tg(igm:eGFP);runx1+/+ to runx1W84X/W84X group, 6 of 8 hosts have runx1+/+-phenotyped B cell reconstitution; and in Tg(igm:eGFP);runx1W84X/W84X mutants to runx1+/+ group, 5 of 7 hosts runx1+/+ displayed B cell phenotype identical to runx1W84X/W84X mutants (figure 4b–d). In brief, these data strongly argued that runx1 regulates development of B cells through a cell-autonomous manner in zebrafish.
Figure 4.
Cell-autonomous regulation of runx1 in B cells development. (a) Schematic outline showing procedure of transplantation. (b) Macroscopic representation of kidney marrow of donors (top), donors after irradiation (middle) and hosts reconstituted with donors kidney morrow (below, 4 weeks after transplantation). Ovals indicate the locations of the head kidneys; dpt, day post transplantation; scales bars, 5 mm. (c) Fluorescent representation of igm:eGFP+ from reconstituted kidney morrow of hosts (4 weeks after transplantation). Scales bars, 100 µm. (d) Percentage of igm:eGFP+ in lymphocytes from reconstituted hosts kidney marrow were calculated by FACS analysis. Each experiment was performed in duplicate. Black boxes outline the captured igm:eGFP+ cells with fluorescence in lymphocytes region of kidney marrow.
3. Discussion
In our study, we confirmed that the necessity of runx1 in B lymphopoiesis of adult zebrafish is conserved. We established the CVID model in adult zebrafish with runx1W84X/W84X mutation, which demonstrates a phenotype consistent with that of adult Runx1-null mice. A decreased ratio of B cells with an increased ratio of T cells was observed in adult zebrafish kidney with runx1 absence. From the reduced igm:eGFP+ and rag2:dsRed+ B cells, we recapitulated that the mature and immature B cells were both decreased in runx1W84X/W84X mutants kidney and spleen. This result could be explained by the previous research that rag2-positive cells are likely to be restricted to immature B cells in the kidney [34]. Furthermore, we found that the absolute T cell number in runx1W84X/W84X mutants is comparable to that of runx1+/+. Nevertheless, we were not surprised to discover the increased percentage of T cells in runx1W84X/W84X mutants. It is supposed that the ratio of T cells may be relatively increased rather than absolutely increased, as it is accompanied by a reduction of B cells.
In mammals, Runx1 is induced to express in early B cell development and maintained at the subsequent various stage of B cells [50–52]. The essential regulators E2a, Ebf1 and Pax5 are tightly associated with maturation of B cells in both mouse and human [35,53]. Our studies showed a marked decrease in the expression of pax5 and ebf1α in runx1W84X/W84X mutants, consistent with previous observations in mice. To our knowledge, runx1 could cooperate with pax5 in activation of the ebf1α gene promoter in mastering the B cells development [20]. We also identified the conserved runx1-ebf1α axis regulation on the B cells development in zebrafish. We confirmed the Runx-binding motifs on ebf1a promoter, which suggested that Runx1 may directly control the transcription of ebf1a in zebrafish. Therefore, in our zebrafish model, runx1 is likely to promote B cell development by regulating key factors such as ebf1a, which in turn regulates pax5, its known downstream target, to control the development of B cells.
Characteristics of the zebrafish immune system are comparable with that of mammals, making it a versatile model to address questions about immunity and disease. By measuring the KLH-mediated proliferation of B cells and T cells in the kidney, we found a negative immune response to antigen and crippled immunoglobulin secretion of B cells in runx1W84X/W84X mutants, suggesting that runx1 deficiency impaired the adaptive defense system. This reminded us that the phenotypes of runx1W84X/W84X mutants were similar to CVID in human. CVID is diagnosed mainly based on decreased serum immunoglobulin levels, recurrent infection and the absence of specific antibodies against antigens [5]. CVID is intractable without clear pathogenesis. To date, it has not been reported that the lesion in runx1 is genetically associated with CVID.
B cells with ineffective V(D)J rearrangements demonstrate immaturity, which promotes apoptosis [54]. Mechanistically, we found that absence of runx1 increased apoptosis of B cells. This is probably due to the blocked maturation of B cells incurred by inhibited runx1 function. In addition, by kidney transplantation, we found cell-autonomous regulation of B cells growth in adult zebrafish. This confirmed the indispensable role of runx1 in haematopoiesis.
In conclusion, loss of runx1 function resulted in dysfunction of B cells in adult zebrafish. We are the first to use inherited runx1W84X/W84X mutants in zebrafish with B cell deficiency to establish a powerful CVID model, which will provide chances to further explore B cell development as well as potential therapy for CVID patients.
4. Material and methods
4.1. Zebrafish strains and maintenance
Zebrafish stocks were handled and bred in standard circulating water system as described previously [55]. runx1W84X/W84X mutant zebrafish line [32,33] was kindly provided by Dr P. Paul Liu and Dr Zilong Wen. runx1+/+ zebrafish were spawned to produce the runx1+/+ zebrafish and homozygous runx1W84X/W84X mutants were spawned to propagate runx1W84X/W84X mutants in this study. Tg(Cau.Ighv-ighm:eGFP) referred to in texts as Tg(igm:eGFP) [35], Tg(rag2:dsRed)zf411 referred to in texts as Tg(rag2:dsRed) [56], Tg(lck:loxp-dsRed-loxp-eGFP) referred to in texts as Tg(lck:dsRed) [25] and Tg(hsp70l:MYC-runx1)hkz02t referred to in texts as hps70l:myc-runx1 [57] were used in this study. Genomic DNA was harvested from clipped tail fins, PCR amplification and digestion by Hae II restriction enzyme determined the genotype of runx1W84X/W84X mutants. Adult runx1W84X/W84X mutants zebrafish were mated to Tg(igm:eGFP), Tg(rag2:dsRed) and Tg(lck:dsRed) respectively, and their embryos were harvested and raised. Then we generated Tg(igm:eGFP);runx1W84X/W84X mutants, Tg(rag2:dsRed);runx1W84X/W84X mutants and Tg(lck:dsRed);runx1W84X/W84X mutants lines. In all experiments, zebrafish embryos were raised in fish water consisting of 2 mg l−1 methylene blue in deionized water as a fungicide.
4.2. Flow cytometry
Haematopoietic cells obtained from adult runx1+/+ and runx1W84X/W84X mutants were processed as described [58]. Haematopoietic cells isolated from kidney and spleen were resuspended using ice-cold PBS with 5% FBS, then subjected to measurement based on forward scatter and side scatter with a flow cytometer (Becton Dickinson, San Jose, CA) and results were analysed with FlowJo software (TreeStar, Ashland, OR). Lymphocytic populations in FSCintSSClow were sorted based on forward and side scatters on flow cytometry sorter (Becton Dickinson, San Jose, CA) and was used in reverse transcription reactions. The percentage of GFP fluorescence-activated cells or dsRed fluorescence-activated cells in the lymphocytic population was analysed [59].
4.3. RNA extraction and quantitative RT-PCR
Total RNA from lymphocytes of adult runx1+/+ and runx1W84X/W84X mutants were extracted by TRIzol Reagent (Roche, Basel, Switzerland) following the manufacturer's instructions and converted to complementary DNA (cDNA) using M-MLV Reverse Transcriptase (Promega, Madison, USA) with oligo18-dT (deoxy-thymine) primers. RNA was treated with RNase-free DNase (Thermo Fisher Scientific, Waltham, USA) before the reverse transcription reaction. Q-RT-PCR reactions were performed using LightCycler Nano System (Roche, Basel, Switzerland) with FastStart Universal SYBR Green Master (ROX) (Roche, Basel, Switzerland) with 10 pmol of each primer and each sample was tested in triplicate. The housekeeping gene, elongation factor 1-α (ef-1α), served as an internal control to normalize the relative fold changes using the ΔΔCt threshold method. The primers used in Q-RT-PCR are listed in table 1.
4.4. Analysis of V(D)J rearrangements
Kidneys were obtained from adult runx1+/+ and runx1W84X/W84X mutants then subjected to genomic DNA isolation (QIAGEN, Hilden, Germany). Semi-nested PCRs were processed with published primers spanning the V(D)J-JM region of igm (igVH1-Jm), and igz (igVH1-Jmz) using the genomic DNA [41–43]. The semi-nested PCRs program was carried out as follows: first round, 1 cycle for 94°C/120 s, 30 cycles for 94°C/30 s, 61°C/30 s and 72°C/30s-90 s, 1 cycle for 72°C/180 s. After the first round of semi-nested PCR, 1 µl PCR product was diluted into 20 times as the template for the second round of semi-nest PCR. The semi-nested PCRs programme of the second round was as follows: 1 cycle for 94°C/120 s, 21 cycles for 94°C/30 s, 61°C/30 s and 72°C/30s-90 s, 1cycle for 72°C/180 s. For tcrβ rearrangements assay, RNA was isolated from the kidney marrow of adult runx1+/+ and mutants and converted to cDNA. tcrβ rearrangements were PCR amplified, referred to in the figure as Vb14.5-Cb1 using semi-nested PCRs, as previously described [42]. The first round of PCR cycle parameters were as follows: 1 cycle for 94°C/120 s, 35 cycles for 94°C/30 s, 56°C/30 s and 72°C/60 s, 1 cycle for 72°C/180 s. 1 µl PCR product was diluted into 20 times as the template for the second round of semi-nest PCR. The cycling parameters were identical to those of the first reaction, except that the steps were repeated for 25 cycles.
The ef-1α and igVH1-V PCR products used as the standard control were run in the parallel tubes. The semi-nested PCR products were analysed by 1.5% agarose gel electrophoresis and stained with ethidium bromide. The primers used in the rearrangement assays are listed in table 2.
4.5. Survey of immune responses
Keyhole limpet haemocyanin (KLH) (Sigma-Aldrich, St. Louis, USA) dissolved in sterile PBS fully, then emulsified in CFA (Sigma-Aldrich, St Louis, USA) and in IFA (Sigma-Aldrich, St Louis, USA) at the concentration of 5 µg µl−1 [35,60]. Ultrasonic emulsification on ice with 4 s, 2 s, 350 W was performed until the mixture would stay agglomerated when dropped on the ice-water. Adult Tg(igm:eGFP);runx1+/+ and Tg(igm:eGFP);runx1W84X/W84X mutants with both sexes, weighing approximately 0.5–1 g, body lengths 2–3 cm were randomly prepared into four groups: the Tg(igm:eGFP);runx1+/+-PBS, the Tg(igm:eGFP);runx1+/+-KLH, the Tg(igm:eGFP);runx1W84X/W84X-PBS and the Tg(igm:eGFP);runx1W84X/W84X -KLH, to receive the detection of immune response. The four groups were immunized by intraperitoneal injection with the same volume of sterile PBS and a dose of 5 µg KLH emulsified in CFA, respectively. Followed 14 days later, the Tg(igm:eGFP);runx1+/+ and Tg(igm:eGFP);runx1W84X/W84X mutants were again intraperitoneally injected with same 1 µl volume of sterile PBS and 5 µg KLH emulsified in IFA, respectively [39]. Four weeks later, the percentage and number of igm:eGFP+ cells in the lymphocytic population of kidney from adult Tg(igm:eGFP);runx1+/+ and Tg (igm:eGFP);runx1W84X/W84X mutants as well as lck:dsRed+ cells in the lymphocytic population of kidney from adult Tg(lck:dsRed);runx1+/+ and Tg(lck:dsRed);runx1W84X/W84X mutation lines were analysed to examine the function of B cells and T cells.
4.6. B cell proliferation and apoptosis assay
Adult runx1+/+ and runx1W84X/W84X mutants were incubated in 10 mM bromodeoxyuridine (BrdU, Sigma-Aldrich, St Louis, USA) dissolved in system water for 4 h as described with modification [61]. Blood smears were obtained as described previously [62]. Kidney marrow blood cells were fixed by 4% paraformaldehyde, stained with mouse-anti-BrdU (Roche, Basel, Switzerland, cat.#1170376001, 1 : 16) and rabbit-anti-dsRed Abs (Clontech, Mountain View, USA, cat.#632496, 1 : 400), coupled with Alexa Fluor 488 anti-mouse (Abcam, England, cat.#ab150153, 1 : 400) and Alexa 555 anti-rabbit (Abcam, England, cat.#ab150078, 1 : 400) for fluorescent observation. The ratio of rag2:dsRed and BrdU co-staining cells in rag2:dsRed+ cells was calculated between runx1+/+ and runx1W84X/W84X mutants. TUNEL assay was conducted using In-situ Cell Death Detection Kit (Roche, Basel, Switzerland) as described [63], coupled with rabbit-anti-dsRed Abs (Clontech, Mountain View, USA, cat.#632496, 1 : 400) and Alexa 555 anti-rabbit (Abcam, England, cat.#ab150078, 1 : 400). The ratio of rag2:dsRed and TUNEL co-staining cells in rag2:dsRed+ cells was calculated between runx1+/+ and runx1W84X/W84X mutants.
4.7. Chromatin immunoprecipitation
Embryos were harvested from hsp70l:myc-runx1zebrafish and heat shocked at 39°C. Crosslinked chromatin was immunoprecipitated with anti-Myc antibody (MBL, Japan, cat.#m192-3, 1 : 63) or IgG (negative control, Invitrogen, USA, cat.#10003D) according to the procedure described by Hart et al. [64]. The immunoprecipitates were subjected to semi-quantitative PCR. The primers used in this assay are listed in table 1.
4.8. Transplantation procedure
Kidney marrow transplantation experiments were carried out as described previously [59,65] with some minor modifications. Adult runx1+/+ and runx1W84X/W84X mutant hosts were γ-irradiated beforehand with a total dose at 25 Gy and then received transplantation after two days without feed. Kidney marrow blood cells suspension from adult donors Tg(igm:eGFP);runx1+/+ and Tg(igm:eGFP);runx1W84X/W84X mutants were filtered through a 40 µm strainer. Kidney marrow blood cells were calculated manually by a haematocytometer. Counted kidney marrow blood cells were centrifuged at 800 g for 5 min at 4°C, then resuspended in injection medium (PBS with 5% FBS containing 3U Heparin and 1U DNaseI) and divided into desired volumes for the following transplantation. Approx. 106 kidney marrow blood cells from donors were transplanted into the heart of hosts using a glass capillary needle (Eppendorf, Hamburg, Germany). Transplanted hosts were raised very carefully and fed for the next week. The three groups assigned were Tg(igm:eGFP);runx1+/+ donor to runx1+/+ host, Tg(igm:eGFP);runx1+/+ donor to runx1W84X/W84X mutants host, Tg(igm:eGFP);runx1W84X/W84X mutants donor to runx1+/+ host. 4 weeks after transplantation, igm:eGFP+ cells and kidneys of hosts were measured by flow cytometry and the fluorescences were observed.
4.9. Imaging analysis
Images of blood smear samples were captured on an Olympus DP 71 microscope (Olympus, Tokyo, Japan) and a Zeiss confocal microscope (ZEISS LSM 510, Germany). Adult zebrafish were observed using a ZEISS microscope (ZEISS Discovery. v. 20, Germany) and photographed by an Olympus MVX10 microscope (Olympus, Tokyo, Japan). All the images were handled by Adobe Photoshop CS5 (Adobe, San Jose).
4.10. Statistical analysis
The data were organized by the two-tailed Student's t-test or ANOVA, while comparison of survival curves was performed with the log-rank test. And the data were shown as a mean ± s.e. of the mean (s.e.m). Differences were considered significant when the P value was less than 0.05. Statistical analyses were done using graphpad Prism v. 6 (GraphPad Software, La Jolla, USA).
Supplementary Material
Acknowledgements
We are grateful for generously providing the runx1W84X/W84X mutant line from Dr P. Paul Liu and Dr Zilong Wen. Tg(Cau.ighv-ighm:eGFP) from Dr David Traver and Tg(lck:loxp-dsRed-loxp-eGFP) from Dr Zilong Wen.
Ethics
All experiments involving zebrafish were done in accordance with the guidelines laid down by the Institutional Animal Care and Use Committee of Southern Medical University.
Data accessibility
All relevant data are included in the manuscript and the electronic supplementary material.
Authors' contributions
Z.H., J.X., Y.Z. and W.Z. designed the experiments; Y.C. and Z.H. designed and performed experiments; Y.C. and X.X. performed the transplantation and immune response assay; Y.C., Z.H., Q.C. and K.C. performed the B cell proliferation and apoptosis assay, and rearrangements assay; Y.C., X.X and Q.C. characterized mutant and performed FACS assay. Y.C., Z.H. and W.Z. wrote the manuscript; and Z.H., J.X., Y.Z. and Z.W. discussed the results and commented on the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81670114); Guangdong Natural Science Foundation (grant no. 2016A030310378).
References
- 1.Barnett BE, Ciocca ML, Goenka R, Barnett LG, Wu J, Laufer TM, Burkhardt JK, Cancro MP, Reiner SL. 2012. Asymmetric B cell division in the germinal center reaction. Science 335, 342–344. ( 10.1126/science.1213495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yasuda T, et al. 2016. Corrigendum: recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat. Genet. 48, 1591 ( 10.1038/ng1216-1587a) [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Cortez VC, del Pino-Molina L, Rodríguez-Ubreva J, Gómez-Cabrero D, Urquiza JM, Tegnér J, Rodríguez-Gallego C, López-Granados E, Ballestar E. 2015. Monozygotic twins discordant for common variable immunodeficiency reveal impaired DNA demethylation during naive-to-memory B-cell transition. Nat. Commun. 6, 7335 ( 10.1038/ncomms8335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boushaki S, et al. 2015. Prevalence of BTK mutations in male Algerian patterns with agammaglobulinemia and severe B cell lymphopenia. Clin. Immunol. 161, 286–290. ( 10.1016/j.clim.2015.09.011) [DOI] [PubMed] [Google Scholar]
- 5.Li J, et al. 2015. Association of CLEC16A with human common variable immunodeficiency disorder and role in murine B cells. Nat. Commun. 6, 6804 ( 10.1038/ncomms7804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes ML, Pridans C, Nutt SL. 2008. The regulation of the B-cell gene expression programme by Pax5. Immunol. Cell Biol. 86, 47–53. ( 10.1038/sj.icb.7100134) [DOI] [PubMed] [Google Scholar]
- 7.Manilay JO, Zouali M. 2014. Tight relationships between B lymphocytes and the skeletal system. Trends Mol. Med. 20, 405–412. ( 10.1016/j.molmed.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 8.Libermann TA, Pan Z, Akbarali Y, Hetherington CJ, Boltax J, Yergeau DA, Zhang DE. 1999. AML1 (CBFalpha2) cooperates with B cell-specific activating protein (BSAP/PAX5) in activation of the B cell-specific BLK gene promoter. J. Biol. Chem. 274, 24 671–24 676. ( 10.1074/jbc.274.35.24671) [DOI] [PubMed] [Google Scholar]
- 9.Kwon K, Hutter C, Sun Q, Bilic I, Cobaleda C, Malin S, Busslinger M. 2008. Instructive role of the transcription factor E2A in early B lymphopoiesis and germinal center B cell development. Immunity 28, 751–762. ( 10.1016/j.immuni.2008.04.014) [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, et al. 2016. Superenhancer reprogramming drives a B-cell-epithelial transition and high-risk leukemia. Genes Dev. 30, 1971–1990. ( 10.1101/gad.283762.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Carlsson L, Grundström T. 2006. Identification of an N-terminal transactivation domain of Runx1 that separates molecular function from global differentiation function. J. Biol. Chem. 281, 25 659–25 669. ( 10.1074/jbc.M603249200) [DOI] [PubMed] [Google Scholar]
- 12.Link KA, Chou FS, Mulloy JC. 2010. Core binding factor at the crossroads: determining the fate of the HSC. J. Cell. Physiol. 222, 50–56. ( 10.1002/jcp.21950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyama S, et al. 2013. Transcription factor RUNX1 promotes survival of acute myeloid leukemia cells. J. Clin. Invest. 123, 3876–3888. ( 10.1172/JCI68557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim W, Barron DA, San Martin R, Chan KS, Tran LL, Yang F, Ressler SJ, Rowley DR. 2014. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. Proc. Natl Acad. Sci. USA 111, 16 389–16 394. ( 10.1073/pnas.1407097111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sood R, Kamikubo Y, Liu P. 2017. Role of RUNX1 in hematological malignancies. Blood 129, 2070–2082. ( 10.1182/blood-2016-10-687830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. 1996. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321–330. ( 10.1016/S0092-8674(00)80986-1) [DOI] [PubMed] [Google Scholar]
- 17.Swiers G, de Bruijn M, Speck NA. 2010. Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int. J. Dev. Biol. 54, 1151–1163. ( 10.1387/ijdb.103106gs) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bresciani E, Carrington B, Wincovitch S, Jones M, Gore AV, Weinstein BM, Sood R, Liu PP. 2014. CBFβ and RUNX1 are required at 2 different steps during the development of hematopoietic stem cells in zebrafish. Blood 124, 70–78. ( 10.1182/blood-2013-10-531988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antony-Debré I, et al. 2012. MYH10 protein expression in platelets as a biomarker of RUNX1 and FLI1 alterations. Blood 120, 2719–2722. ( 10.1182/blood-2012-04-422352) [DOI] [PubMed] [Google Scholar]
- 20.Seo W, Ikawa T, Kawamoto H, Taniuchi I. 2012. Runx1-Cbfbeta facilitates early B lymphocyte development by regulating expression of Ebf1. J. Exp. Med. 209, 1255–1262. ( 10.1084/jem.20112745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niebuhr B, et al. 2013. Runx1 is essential at two stages of early murine B-cell development. Blood 122, 413–423. ( 10.1182/blood-2013-01-480244) [DOI] [PubMed] [Google Scholar]
- 22.Zaliova M, et al. 2017. ETV6/RUNX1-like acute lymphoblastic leukemia: a novel B-cell precursor leukemia subtype associated with the CD27/CD44 immunophenotype. Genes Chromosomes Cancer 56, 608–616. ( 10.1002/gcc.22464) [DOI] [PubMed] [Google Scholar]
- 23.Grossmann V, Kern W, Harbich S, Alpermann T, Jeromin S, Schnittger S, Haferlach C, Haferlach T, Kohlmann A. 2011. Prognostic relevance of RUNX1 mutations in T-cell acute lymphoblastic leukemia. Haematologica 96, 1874–1877. ( 10.3324/haematol.2011.043919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Y, et al. 2017. The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells. J. Exp. Med. 214, 3347–3360. ( 10.1084/jem.20170488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwanami N. 2014. Zebrafish as a model for understanding the evolution of the vertebrate immune system and human primary immunodeficiency. Exp. Hematol. 42, 697–706. ( 10.1016/j.exphem.2014.05.001) [DOI] [PubMed] [Google Scholar]
- 26.Yan B, Han P, Pan L, Lu W, Xiong J, Zhang M, Zhang W, Li L, Wen Z. 2014. IL-1beta and reactive oxygen species differentially regulate neutrophil directional migration and Basal random motility in a zebrafish injury-induced inflammation model. J. Immunol. 192, 5998–6008. ( 10.4049/jimmunol.1301645) [DOI] [PubMed] [Google Scholar]
- 27.Willett CE, Cortes A, Zuasti A, Zapata AG. 1999. Early hematopoiesis and developing lymphoid organs in the zebrafish. Dev. Dyn. 214, 323–336. ( 10.1002/(SICI)1097-0177(199904)214:4%3C323::AID-AJA5%3E3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- 28.Trede NS, Zapata A, Zon LI. 2001. Fishing for lymphoid genes. Trends Immunol. 22, 302–307. ( 10.1016/S1471-4906(01)01939-1) [DOI] [PubMed] [Google Scholar]
- 29.Rijkers GT, Frederix-Wolters EM, van Muiswinkel WB. 1980. The immune system of cyprinid fish. Kinetics and temperature dependence of antibody-producing cells in carp (Cyprinus carpio). Immunology 41, 91–97. [PMC free article] [PubMed] [Google Scholar]
- 30.Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. 2002. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 111, 621–633. ( 10.1016/S0092-8674(02)01111-X) [DOI] [PubMed] [Google Scholar]
- 31.Palmi C, et al. 2014. Cytoskeletal regulatory gene expression and migratory properties of B-cell progenitors are affected by the ETV6-RUNX1 rearrangement. Mol. Cancer Res. 12, 1796–1806. ( 10.1158/1541-7786.MCR-14-0056-T) [DOI] [PubMed] [Google Scholar]
- 32.Jin H, Sood R, Xu J, Zhen F, English MA, Liu PP, Wen Z. 2009. Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI. Development 136, 647–654. ( 10.1242/dev.029637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sood R, et al. 2010. Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation. Blood 115, 2806–2809. ( 10.1182/blood-2009-08-236729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langenau DM, Ferrando AA, Traver D, Kutok JL, Hezel JP, Kanki JP, Zon LI, Look AT, Trede NS. 2004. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl Acad. Sci. USA 101, 7369–7374. ( 10.1073/pnas.0402248101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page DM, et al. 2013. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood 122, e1–e11. ( 10.1182/blood-2012-12-471029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukin K, Fields S, Lopez D, Cherrier M, Ternyak K, Ramírez J, Feeney AJ, Hagman J. 2010. Compound haploinsufficiencies of Ebf1 and Runx1 genes impede B cell lineage progression. Proc. Natl Acad. Sci. USA 107, 7869–7874. ( 10.1073/pnas.1003525107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Wu Y, Hu Q, Li Y. 2015. Differences on the biological function of three Ig isotypes in zebrafish: a gene expression profile. Fish Shellfish Immunol. 44, 283–286. ( 10.1016/j.fsi.2015.02.030) [DOI] [PubMed] [Google Scholar]
- 38.Ungerbäck J, Ahsberg J, Strid T, Somasundaram R, Sigvardsson M. 2015. Combined heterozygous loss of Ebf1 and Pax5 allows for T-lineage conversion of B cell progenitors. J. Exp. Med. 212, 1109–1123. ( 10.1084/jem.20132100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin AF, Xiang LX, Wang QL, Dong WR, Gong YF, Shao JZ. 2009. The DC-SIGN of zebrafish: insights into the existence of a CD209 homologue in a lower vertebrate and its involvement in adaptive immunity. J. Immunol. 183, 7398–7410. ( 10.4049/jimmunol.0803955) [DOI] [PubMed] [Google Scholar]
- 40.Kuehn HS, et al. 2016. Loss of B cells in patients with heterozygous mutations in IKAROS. N. Engl. J. Med. 374, 1032–1043. ( 10.1056/NEJMoa1512234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wienholds E, Schulte-Merker S, Walderich B, Plasterk RH. 2002. Target-selected inactivation of the zebrafish rag1 gene. Science 297, 99–102. ( 10.1126/science.1071762) [DOI] [PubMed] [Google Scholar]
- 42.Schorpp M, Bialecki M, Diekhoff D, Walderich B, Odenthal J, Maischein HM, Zapata AG, Boehm T. 2006. Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J. Immunol. 177, 2463–2476. ( 10.4049/jimmunol.177.4.2463) [DOI] [PubMed] [Google Scholar]
- 43.Petrie-Hanson L, Hohn C, Hanson L. 2009. Characterization of rag1 mutant zebrafish leukocytes. Bmc Immunol. 10, 8 ( 10.1186/1471-2172-10-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J, et al. 2013. Suppression of Pu.1 function results in expanded myelopoiesis in zebrafish. Leukemia 27, 1913–1917. ( 10.1038/leu.2013.67) [DOI] [PubMed] [Google Scholar]
- 45.Györy I, Boller S, Nechanitzky R, Mandel E, Pott S, Liu E, Grosschedl R. 2012. Transcription factor Ebf1 regulates differentiation stage-specific signaling, proliferation, and survival of B cells. Genes Dev. 26, 668–682. ( 10.1101/gad.187328.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandel EM, Grosschedl R. 2010. Transcription control of early B cell differentiation. Curr. Opin. Immunol. 22, 161–167. ( 10.1016/j.coi.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 47.Parker L, Stainier DY. 1999. Cell-autonomous and non-autonomous requirements for the zebrafish gene cloche in hematopoiesis. Development 126, 2643–2651. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, et al. 2013. Fev regulates hematopoietic stem cell development via ERK signaling. Blood 122, 367–375. ( 10.1182/blood-2012-10-462655) [DOI] [PubMed] [Google Scholar]
- 49.Hess I, Boehm T. 2016. Stable multilineage xenogeneic replacement of definitive hematopoiesis in adult zebrafish. Sci. Rep. 6, 19634 ( 10.1038/srep19634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chimienti G, Alaibac M, Marzullo F, Carbone A, Pepe G. 2000. The expression pattern of the AML1 gene in non-Hodgkin's B-cell lymphomas and normal B lymphocytes. Blood Cells Mol. Dis. 26, 186–192. ( 10.1006/bcmd.2000.0295) [DOI] [PubMed] [Google Scholar]
- 51.Bäsecke J, Feuring-Buske M, Brittinger G, Schaefer UW, Hiddemann W, Griesinger F. 2002. Transcription of AML1 in hematopoietic subfractions of normal adults. Ann. Hematol. 81, 254–257. ( 10.1007/s00277-002-0453-8) [DOI] [PubMed] [Google Scholar]
- 52.Lorsbach RB, Moore J, Ang SO, Sun W, Lenny N, Downing JR. 2004. Role of RUNX1 in adult hematopoiesis: analysis of RUNX1-IRES-GFP knock-in mice reveals differential lineage expression. Blood 103, 2522–2529. ( 10.1182/blood-2003-07-2439) [DOI] [PubMed] [Google Scholar]
- 53.Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M. 2007. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 27, 49–63. ( 10.1016/j.immuni.2007.05.019) [DOI] [PubMed] [Google Scholar]
- 54.Lam QL, Lo CK, Zheng BJ, Ko KH, Osmond DG, Wu GE, Rottapel R, Lu L. 2007. Impaired V(D)J recombination and increased apoptosis among B cell precursors in the bone marrow of c-Abl-deficient mice. Int. Immunol. 19, 267–276. ( 10.1093/intimm/dxl143) [DOI] [PubMed] [Google Scholar]
- 55.Westerfield M. 1995. The zebrafish book: a guide for the laboratory use of zebrafish Danio (Brachydanio) rerio. Oregon, OR: USA: Oregon University Press. [Google Scholar]
- 56.Willett CE, Cherry JJ, Steiner LA. 1997. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics 45, 394–404. ( 10.1007/s002510050221) [DOI] [PubMed] [Google Scholar]
- 57.Jin H, Li L, Xu J, Zhen F, Zhu L, Liu PP, Zhang M, Zhang W, Wen Z. 2012. Runx1 regulates embryonic myeloid fate choice in zebrafish through a negative feedback loop inhibiting Pu.1 expression. Blood 119, 5239–5249. ( 10.1182/blood-2011-12-398362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stachura DL, Traver D. 2016. Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol. 133, 11–53. ( 10.1016/bs.mcb.2016.03.022) [DOI] [PubMed] [Google Scholar]
- 59.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. 2003. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246. ( 10.1038/ni1007) [DOI] [PubMed] [Google Scholar]
- 60.Weir H, Chen PL, Deiss TC, Jacobs N, Nabity MB, Young M, Criscitiello MF. 2015. DNP-KLH yields changes in leukocyte populations and immunoglobulin isotype use with different immunization routes in zebrafish. Front. Immunol. 6, 606 ( 10.3389/fimmu.2015.00606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. 2005. Intestinal growth and differentiation in zebrafish. Mech. Dev. 122, 157–173. ( 10.1016/j.mod.2004.10.009) [DOI] [PubMed] [Google Scholar]
- 62.Balla KM, Lugo-Villarino G, Spitsbergen JM, Stachura DL, Hu Y, Banuelos K, Romo-Fewell O, Aroian RV, Traver D. 2010. Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood 116, 3944–3954. ( 10.1182/blood-2010-03-267419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du L, Xu J, Li X, Ma N, Liu Y, Peng J, Osato M, Zhang W, Wen Z. 2011. Rumba and Haus3 are essential factors for the maintenance of hematopoietic stem/progenitor cells during zebrafish hematopoiesis. Development 138, 619–629. ( 10.1242/dev.054536) [DOI] [PubMed] [Google Scholar]
- 64.Hart DO, Raha T, Lawson ND, Green MR. 2007. Initiation of zebrafish hematopoiesis by the TATA-box-binding protein-related factor, Trf3. Nature 450, 1082–1085. ( 10.1038/nature06349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W, et al. 2017. c-myb hyperactivity leads to myeloid and lymphoid malignancies in zebrafish. Leukemia 31, 222–233. ( 10.1038/leu.2016.170) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the manuscript and the electronic supplementary material.