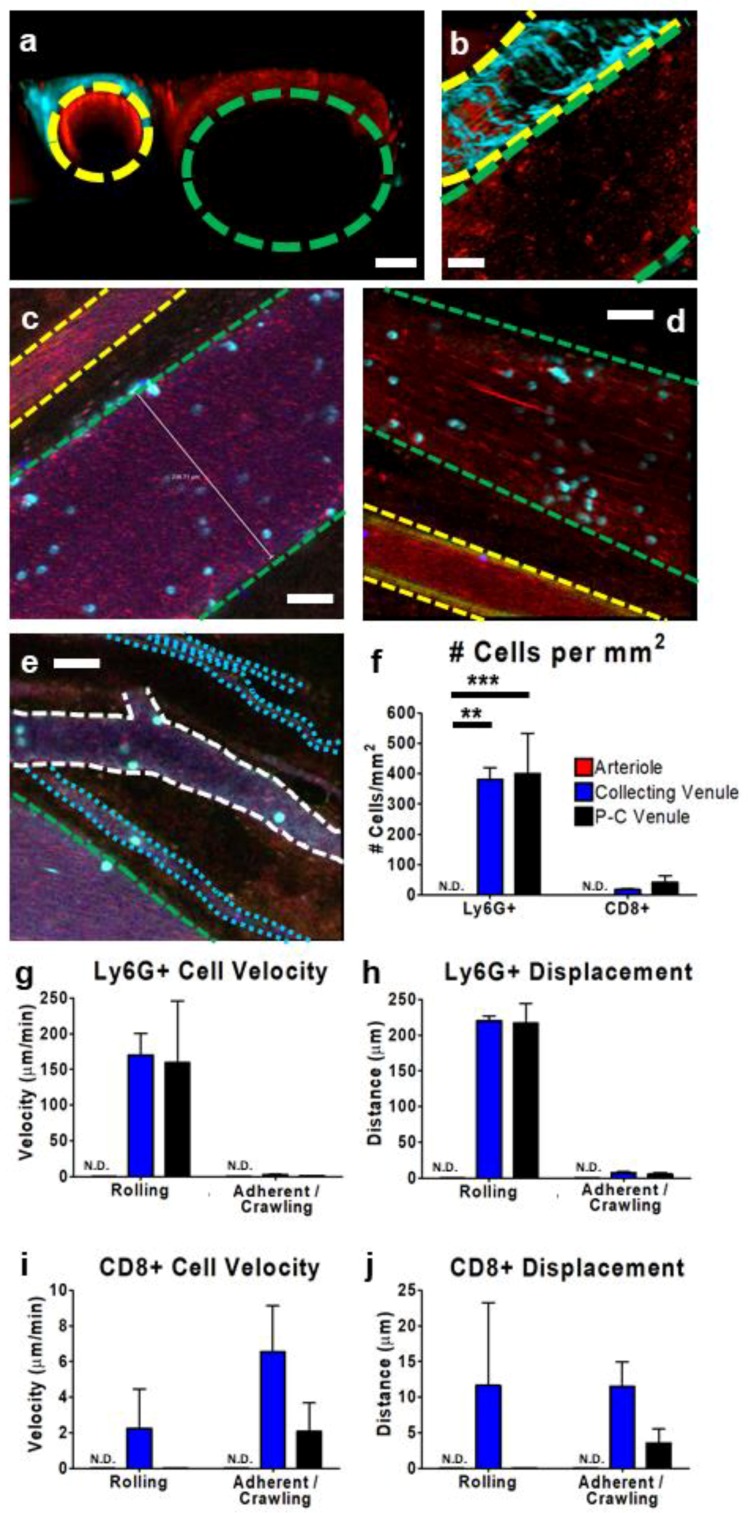
Figure 3.
Characterization of tumor vasculature and leukocyte behavior within subcutaneous CT-26 tumor vessels. Representative IVM images (a–e) show the tumor vasculature. Using multiphoton imaging (a,b), differentiation between arterioles (yellow outline) and collecting venules (green outline) was facilitated by the observation of a collagen sheath (cyan-colored second-harmonic generation) surrounding the arteriole. Vasculature was highlighted by the presence of circulating platelets (red; PE-conjugated anti-CD49b), neutrophils (cyan; BV421-conjugated Ly6G), and CD8+ leukocytes (blue; eFluor 660-conjugated anti-CD8) and was imaged in either cross section (a) or transverse section (b). Using resonant scanning confocal microscopy (c–e), arterioles (yellow outline) were apparent as a result of increased autofluorescence (green) and collecting venules (green outline) were seen as parallel unbranching structures. In contrast, post-capillary venules (P-C venules) were observed as narrower, branching vessels (white outline), and tumor microcirculation/capillaries as very narrow (1–2 cell diameter) vessels (cyan outline) that followed a more convoluted path. Quantification of neutrophil (Ly6G+) and cytotoxic T cell (CD8+) interactions (cells present for ≥3 min) within arterioles (red), collecting venules (blue), and post-capillary venules (black) (f). Cell velocity (g,i) and displacement (h,j) of rolling and adherent and crawling neutrophils (g,h) and CD8+ T cells (i,j), as measured over a 10 min imaging period in a subcutaneous CT-26 tumour; n = 3 animals. Data displayed as the mean ± SEM. Total cell counts normalized for the area of each image occupied by a given vessel type. The white scale bar represents 50 µm. Statistical significance was determined using ANOVA; ** = p < 0.01; *** = p < 0.001; N.D. = not detected. Images in (c–e) were capture using resonant-scanning confocal microscopy, whereas images in (a,b) were captured using resonant scanning multiphoton microscopy.