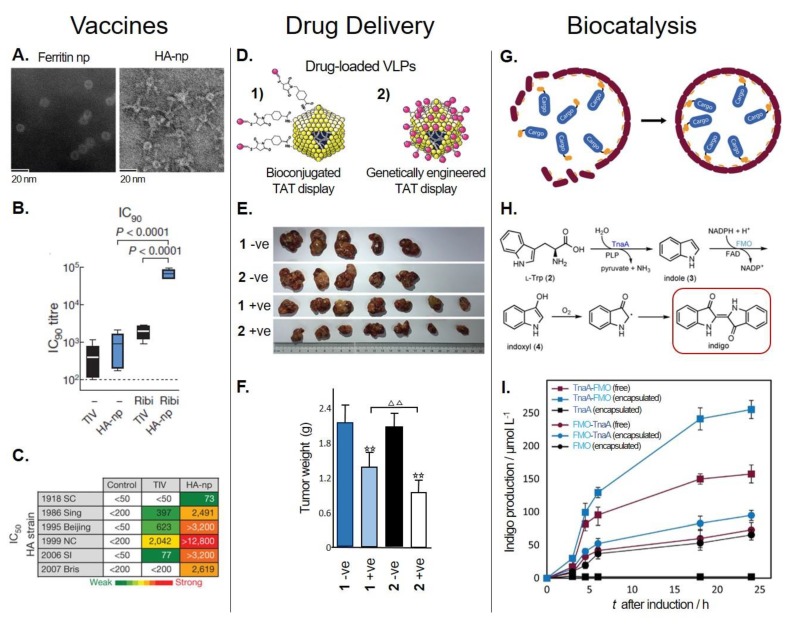
Figure 3.
Examples of the bioapplications of PNPs. (Left panel) Vaccines: Development of a PNP-based influenza vaccine. (A) Transmission electron microscopy (TEM) image showing unmodified ferritin nanoparticles (np) (left) and modified ferritin np with visible hemagglutinin (HA) spikes (HA-np) (right). (B) Comparison of the immunogenicity (in mice) of trivalent inactivated influenza vaccine (TIV, control) or HA-np, without (−) or with adjuvant (Ribi). Neutralization titers (IC90) were determined by measuring the concentration of antibody required to inhibit viral entry by 90%. (C) Table showing the neutralization (IC50 values) of immune sera induced by TIV or HA-np (with Ribi) against a range of H1N1 pseudotyped influenza viruses. Heat map is a colored gradient, from green (weak) to yellow to red (strong), reflecting the neutralization strength. Adapted with permission of [162]. (Middle panel) Drug delivery: A PNP-based microRNA delivery system for targeted cancer therapy. (D) MS2 VLPs loaded with an anticancer microRNA (miR122) and modified to display a cell-penetrating peptide (TAT) by either (1) bioconjugation (crosslinking); or (2) genetic engineering. (E) Tumors isolated from mouse models of hepatocellular carcinoma (HCC) after three weeks of treatment with either of the modified VLPs, which were loaded with miR122 (+ve) or a noncoding miRNA control (−ve). (F) Tumor weights after treatment with modified VLPs. Data compared with their negative controls (−ve) are represented by: ☆☆ p < 0.01; data from the genetically modified VLPs (2 +ve) treated group compared with the bioconjugated (crosslinked) VLPs treated group (1 +ve) are represented by: ΔΔ p < 0.01. Adapted with permission of [92]. (Right panel) Biocatalysis: In vivo loading of multiple enzymes inside PNPs for biocatalysis. (G) Genetic incorporation of the SpyCatcher/SpyTag system into the internal cavity of the MS2 VLP, which facilitates the loading of cargo tagged with either SpyCatcher or SpyTag during MS2 self-assembly in vivo. (H) Diagram of the sequential two-enzyme (i.e., pyridoxal phosphate (PLP)-dependent tryptophanase (TnaA) and flavin mononucleotide and nicotinamide adenine dinucleotide phosphate (NADPH)-dependent monooxygenase (FMO)) biosynthetic pathway for the production of indigo from l-tryptophan (l-Trp). (I) In vivo indigo production of E. coli strains expressing either of the encapsulated polycistronic operons (containing both enzymes): “TnaA + FMO” or “FMO + TnaA”, and their respective controls: free and single encapsulated enzymes. Adapted with permission of [139].