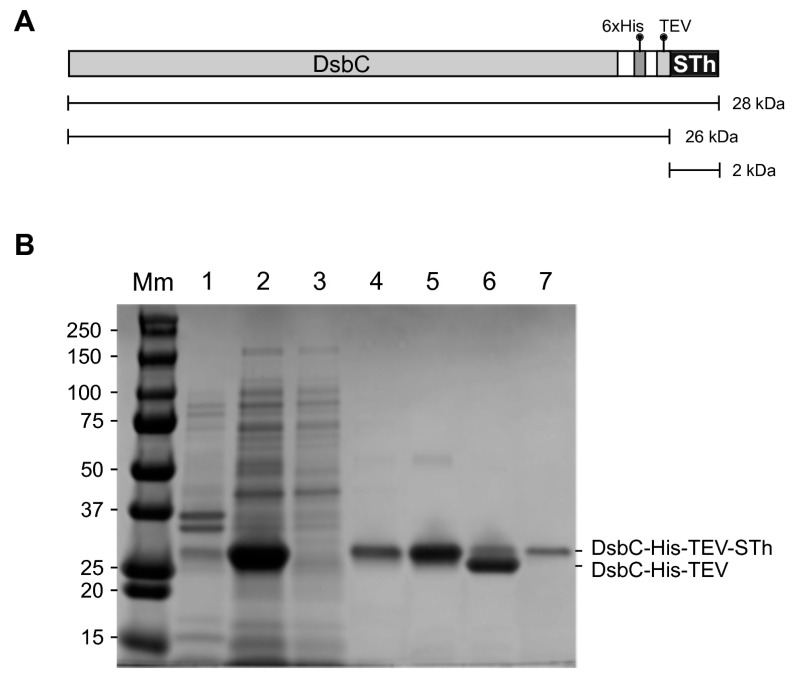
Figure 1.
Purification of the DsbC-His-TEV-STh fusion protein and release of free STh peptide. (A) Cartoon of the DsbC-His-TEV-STh fusion protein with molecular mass of the full-sized fusion protein and those of the products after cleavage by the TEV protease: DsbC-His-TEV and STh. (B) SDS-PAGE gel showing expression of the DsbC-His-TEV-STh fusion protein in E. coli and the different steps of the purification protocol. Molecular masses (kDa) of the protein standard are shown to the left. Lane Mm: Molecular marker. Lane 1: resuspended pellet after sonication, centrifugation to remove cell debris, and removal of the clarified lysate. Lane 2: clarified lysate after sonication and centrifugation to remove debris. Lane 3: Ni-NTA agarose column flowthrough. Lane 4: wash. Lane 5: eluate from the Ni-NTA agarose column after buffer exchange. Lane 6: sample after overnight cleavage with TEV protease. Lane 7: TEV protease; note that it migrates with the same apparent mass as the full-length fusion protein. Both the fusion protein and TEV are removed in a second Ni-NTA agarose purification step (not shown). The STh peptide was collected in the flowthrough. The 2 kDa STh peptide is not visible on the gel. The location of the full-length and cleaved fusion proteins is indicated to the right.