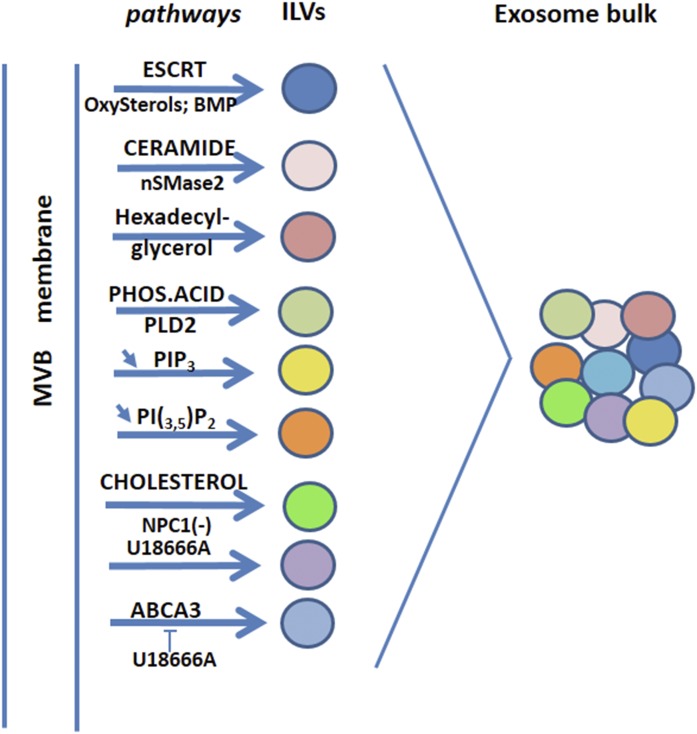
Fig. 2.
Lipid pathways involved in exosome biogenesis processes. Connections with lipid pathways are present within the ESCRT, the first pathway described for ILV biogenesis (38). Indeed, Vps4, a member of the AAA family of proteins (ATPases associated with a variety of activities), interacts with oxysterol binding proteins (Osh6 and Osh7) (6), and Alix interacts with BMP via a specific sequence domain (8). Independent of the ESCRT machinery are lipid pathways involving ceramides (nSMase2) (46) and, to some extent, phosphatidic acid (PHOS.ACID/PLD2) (11). Another neutral lipid, precursor of ether-linked phospholipids, hexadecyl-glycerol, stimulates exosome production (88). The phosphoinositides, PIP3 and PI(3,5)P2, act in a negative way because inhibition of PI3K/Akt (25, 42) and PIKfyve (86) favor exosome production. Because PI3K is involved in macroautophagy (89), this observation indicates that there is a requirement to block macroautophagy to promote the microautophay process involved in exosome biogenesis (90). Indeed, rapamycin, the inhibitor of mammalian target of rapamycin (mTOR)C1 induces macroautophagy (90, 91) and decreases exosome production (84). However an alternative pathway for exosome production via a secretory autophagy process, distinct from the degradative autophagy, has been reported following PIKfyve inhibition (86). Because PIKfyve activity promotes mTORC1 translocation to the plasma membrane (92), the reverse PIKfyve inhibition might retain mTORC1 on the late endosome membrane. mTORC1 is located on the endosome membrane (93) and could have a direct function at this location to generate ILVs. mTORC1 is activated by the endosomal cholesterol (93) and enhanced exosome production was reported when cholesterol was supplied to glial cells either directly or by means of U18666A (94). mTORC1 activation by cholesterol is reversed by NPC1, which effluxes cholesterol from endosomes (93). Consistently, NPC1 inactivation is required to enhance exosome production (94). U18666A displays an opposite effect on lymphoblastoma cells where it inhibits exosome production, which is mediated in these cells by the lipid transporter, ABCA3 (84). It is worth noting that NPC1 and ABCA3-containing endosomes are distinct populations (95). In addition, the effect of U18666A cannot be restricted to cholesterol because U18666A also promotes the accumulation of oxysterols in cells (cholesterol 5,6 epoxides) and cholesterol precursors (zymostenol, desmosterol) (96). In the figure, each pathway leading to a specific vesicle content is a graphical simplification. Combination of the various pathways reported could depend upon cell type and activation conditions or mobilization of distinct late endosome populations, and several pathways can probably be triggered at the same time.