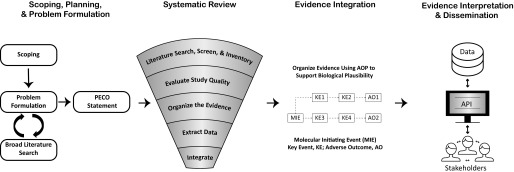
Figure 2.
Integration of the Adverse Outcome Pathway (AOP) framework into the risk-assessment process. The figure above depicts how the AOP framework could be used to organize study data supporting a scientific assessment. Although a systematic literature search was not performed in this example, a protocol including exposure and end point effects of interest (such as epigenetic modification) can be included in the scoping, planning, and problem formulation phase (column 1) and implemented during the systematic review (column 2). Relevant study evidence could then be assessed for quality and organized using an AOP framework (column 3) to improve interpretation. This workflow could build confidence in the assessment process by efficiently and transparently disseminating scientific information, perhaps through a public database accessible to stakeholders through an application programming interface.