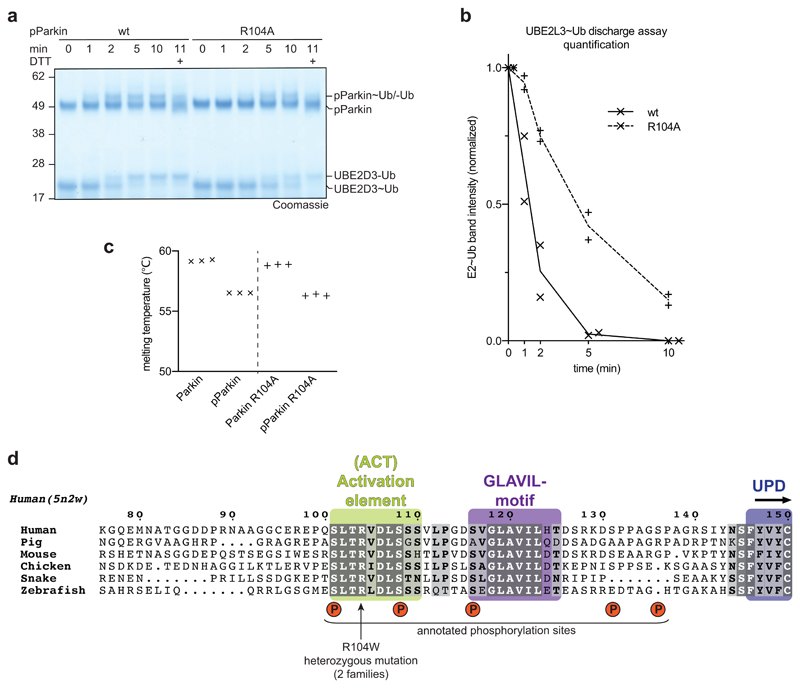
Extended Data Figure 8. A regulatory role of the Parkin Ubl-UPD linker.
a,b, E2 discharge assay resolved on a Coomassie stained SDS-PAGE gel (a) and quantified from band intensities (b) for phospho-Parkin and phospho-Parkin R104A. This is representative of at least two independent experiments, for gel source data, see Supplementary Fig. 1. The mutation in the ACT element leads to lower discharge activity, suggesting that the residue is required to dislodge RING2 from the Parkin core. c, Parkin R104A is equally stable as compared to wild-type Parkin, in the unphosphorylated or phosphorylated form. Thermal denaturation experiments were performed as technical triplicate.
d, Sequence detail of the Ubl-UPD linker, which contains the here described ACT element. In the ACT element as bound to phospho-Parkin-pUb, the positions for two annotated (in PhosphoSitePlus) Parkin phosphorylation sites, Ser101 and Ser108, are resolved. Phosphorylation of Ser101 decreases Parkin activity41, which is likely explained by phosphorylation preventing phospho-Ubl and/or linker binding to the UPD. It is hence highly likely that phosphorylation of Parkin on these residues provides additional layers of Parkin regulation that remain to be uncovered in future work. As an example, Parkin phosphorylation by PKA was recently reported to be a mechanism of Parkin inhibition in beige-to-white adipocyte transition, although phosphorylation sites remained unclear 42.
Residues before the ACT element (aa 73-99), and after the ACT element, (aa 109-142) are disordered in our structure. The last ordered residue, Ser108, is tantalisingly close to the REP binding site as well as to the phospho-ubiquitin binding pocket, but disorder suggests that clear binding sites for other conserved linker residues, in particular for the Parkin GLAVIL motif, are not present. HDX-MS also does not reveal additional protection of the linker, even when the E2~Ub conjugate is bound, suggesting that the GLAVIL motif may not bind the E2 (Fig. 1, Extended Data Fig. 2, 3). On the other hand, there are at least three additional annotated phosphorylation sites, Ser116, Ser131 and Ser13615,41,43,44, suggesting that the second part of the linker may also be regulated. Phosphorylation on these residues could change its ability of the disordered parts of the linker to interact with Parkin in cis. For example, we would speculate that a phosphorylated Ser116 could e.g. reach the phosphate binding pocket occupied by phospho-Ser65 of ubiquitin. Alternatively, the remaining Ubl-UPD linker may be important for substrate recruitment, or involved in other, PINK1-independent mechanisms of Parkin activation.