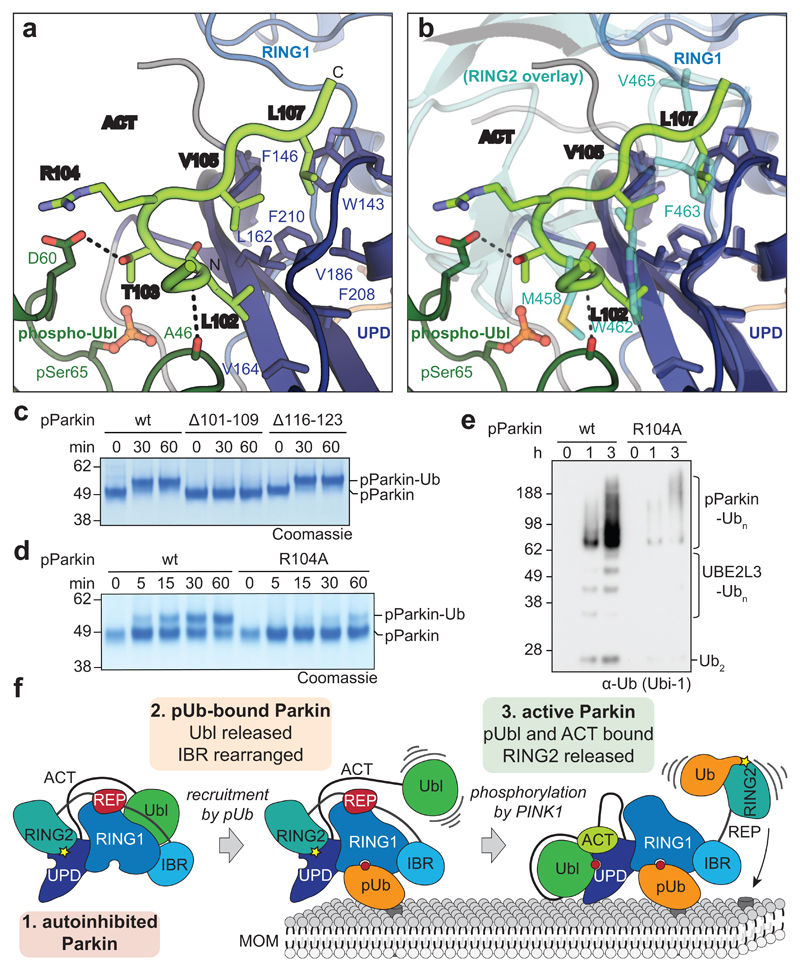
Figure 4. An Activating element (ACT) in Parkin.
a, Structural detail of the ordered Activating element (ACT) within the Parkin phospho-Ubl-UPD linker. Three hydrophobic ACT residues bind the hydrophobic UPD groove, and polar ACT residues contact the phospho-Ubl. b, Superposition of the ACT with RING2 (5n2w, 6, semi-transparent) in the same orientation as in a. Hydrophobic ACT residues mimic RING2 interactions. c, Ub-VS charging assay of phospho-Parkin, and phospho-Parkin variants lacking the ACT (∆101-109) or the second conserved hydrophobic linker sequence (∆116-123). Experiments were performed in duplicate with identical results, for gel source data, see Supplementary Fig. 1. d, Ub-VS charging assay as in c, for phospho-Parkin wild-type (wt) or R104A mutant. Patients with Parkin R104W suffer from AR-JP31. Experiments were performed in duplicate with identical results, for gel source data, see Supplementary Fig. 1. e, Activity of phospho-Parkin wild-type (wt) and R104A in vitro, with UBE2L3 as the E2 enzyme. The reaction was resolved by SDS-PAGE and Western blotted for ubiquitin. A representative gel of three independent experiments is shown. For source data, see Supplementary Fig. 1. f, Model of the sequential domain rearrangements required for full Parkin activation, extended from 3 (also see Fig. 1a). In autoinhibited Parkin, the Ubl, REP and RING2 assume inhibitory positions. Phospho-ubiquitin binding induces MOM localisation, repositioning of the IBR domain and release of the Ubl domain. Phosphorylation of Parkin allows the phospho-Ubl domain and ACT element to bind to the UPD, to replace and release the RING2 and REP, enabling MOM protein ubiquitination.