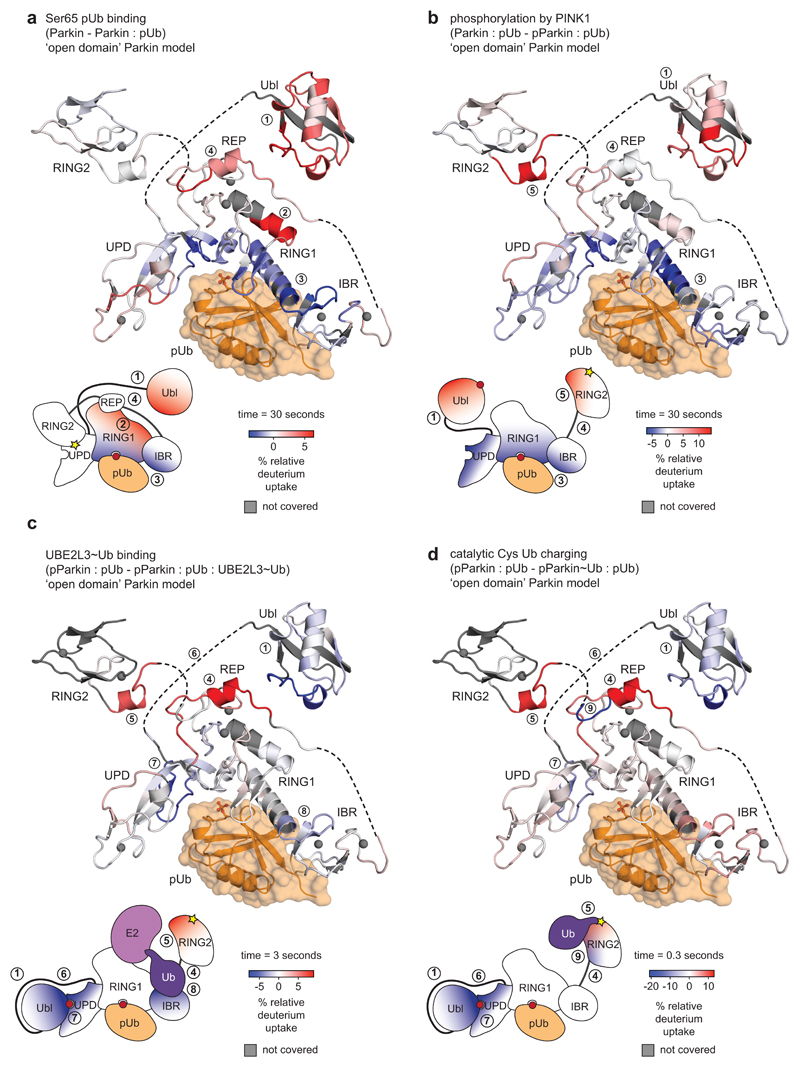
Extended Data Figure 3. Graphical representation of HDX-MS data.
Data from HDX-MS experiments (Fig. 1b-e) was plotted onto a stylised ‘open domain’ model of Parkin, with identical colouring (blue, more protected from solvent exchange compared to previous state; red less protected from solvent exchange compared to previous state). Grey regions correspond to peptides that were not covered or could not be analysed due to modification.
Schematic domain representations indicate an average change of the corresponding interfaces across all time points. White regions indicate no change.
a, Parkin compared to Parkin:phospho-ubiquitin.
b, Parkin:phospho-ubiquitin compared to phospho-Parkin:phospho-ubiquitin
c, phospho-Parkin:phospho-ubiquitin compared to phospho-Parkin:phospho-ubiquitin in complex with UBE2L3-Ub isopeptide UBE2L3~Ub thioester mimetic (see Online Methods). This experiment confirmed a previously reported binding site for the E2-conjugated ubiquitin on the RBR6,29 (8).
d, phospho-Parkin:phospho-ubiquitin compared to Ub-VS-reacted phospho-Parkin:phospho-ubiquitin. Reaction with Ub-VS leads to modification of the catalytic Cys431 containing-peptide, generating non-identical peptides precluding comparison by HDX-MS. Low coverage of the RING2 domain can be explained by ubiquitin resistance to pepsin cleavage, leading to protection of the linked RING2 domain and subsequent peptide loss. To allow comparison, these peptides were also omitted form analysis of the UBE2L3 sample.
In c and d, the structure representation is deceiving since REP and RING2 are highly mobile and are no longer bound to the Parkin core. Indeed, the high HD exchange in the REP sequence in active Parkin (see Fig. 1d, e, peptide (4) in Extended Data Fig. 2d) indicates an additional loss of secondary structure in this helical element when REP and RING2 are released.