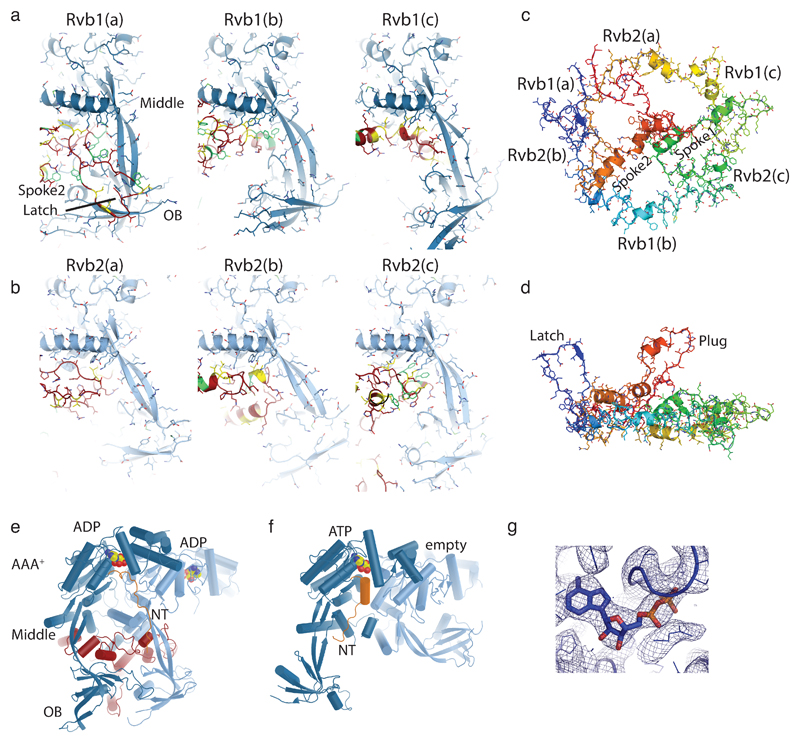
Extended Data Figure 5. Details of Rvb1/2-Ino80insert interactions.
(a) Close-up views of Rvb1 client cavities (blue), bound to the different interaction elements of Ino80insert (red, with yellow hydrophobic and green aromatic side chains).
(b) Same as (a), but depicting Rvb2 client cavities
(c) Ino80insert shown in rainbow coloring (red: N-terminus, blue C-terminus) to highlight the circular fold. Selected elements as well as the positions of the Rvb1/2 binding partners are annotated.
(d) Same as (c) but viewed from the side to highlight the protruding “plug” and “latch” elements
(e) Rvb1/2 pair (pair “c” from the hexamer in Fig. 1c) bound to Ino80insert in comparison with
(f) a Rvb1/2 pair from the unliganded dodecameric state (PDB: 4wvy). The comparison shows how client binding arranges the AAA+, OB and middle layers and displaces the N-terminal domain of Rvb1 from the client pocket, seen also for hINO80core 23. Both types of conformational changes impact on the ADP binding site (ADP, ATP: color coded spheres), suggesting how client interactions are allosterically coupled to ATPase activity of Rvb1/2.
(g) Exemplary view of the ADP coordination along with the superimposed map.