Abstract
Atherosclerosis (AS) is a cardiovascular disease with a relatively high incidence rate. Krüppel-like factor 15 (KLF15) has a role in numerous pathological processes, including nephropathy, abnormal glucose metabolism and myocardial injury. The aim of the present study was to investigate the function of KLF15 in vascular endothelial dysfunction. MTT analyses, nitric oxide (NO) detection and cell adhesion detection kits were used to investigate the viability and adhesion of, and quantity of NO released by Eahy926 cells induced by tumor necrosis factor (TNF)-α, respectively. Reverse transcription-quantitative polymerase chain reaction and western blot analyses were performed to determine the expression levels of KLF15, endothelial nitric oxide synthase, monocyte chemoattractant protein-1 (MCP-1), intercellular adhesion molecule-1 (ICAM-1), transforming growth factor-β1 (TGF-β1), phosphorylated (p-)transcription factor p65 (p65) and nuclear factor erythroid 2-related factor 2 (Nrf2). The results of the present study demonstrated that TNF-α was able to induce vascular endothelial dysfunction in Eahy926 cells at an optimum concentration of 10 ng/ml. Overexpression of KLF15 markedly enhanced cell viability in addition to the quantity of released NO of TNF-α-induced Eahy926 cells, and increased the expression levels of eNOS and Nrf2. Furthermore, overexpression of KLF15 markedly suppressed the rate of cellular adhesion, and downregulated levels of MCP-1, ICAM-1, TGF-β1 and p-p65 in TNF-α induced Eahy926 cells. In conclusion, the results of the present study suggested that overexpression of KLF15 in Eahy926 cells exhibited a protective effect against TNF-α induced dysfunction via activation of Nrf2 signaling and inhibition of nuclear factor κB signaling.
Keywords: Krüppel-like factor 15, dysfunction, atherosclerosis, nuclear factor-κB signaling
Introduction
In recent years, obesity has been revealed to be closely associated with metabolic abnormalities, which represents a risk factor for the development of atherosclerosis (AS), cardiovascular disease, cancer and other diseases (1). Metabolically healthy but obese (MHO) is an obesity subgroup, which is characterized by obesity and high insulin sensitivity, and accounts for 20–30% of patients with obesity worldwide (2,3). Similarly, MHO may cause various vascular diseases, including AS, cerebral infarction and large artery embolism, which are induced by dysfunction of the vascular endothelium (4,5).
AS is a cardiovascular disease, which exhibits a relatively high incidence rate and may subsequently induce arterial thrombosis in acute coronary syndromes, strokes and various other diseases, which may pose a threat to human mortality (6). According to a previous study, the pathogenesis of AS is highly complex (7). It has been widely established that endothelial dysfunction is an important factor in the early stage of AS (8). Endothelial dysfunction results in functional cell alterations, and may be characterized by the suppressed release of nitric oxide (NO) and NO bioavailability, in addition to the enhanced expression of adhesion molecules and chemokines (9). Interaction between of these alterations and smooth muscle cells located in blood vessels in turn alters vascular function and structure, which ultimately leads to AS (8,9). Therefore, attenuation of endothelial dysfunction may reduce the risk of the development of AS.
Krüppel-like factors (KLFs) are a class of zinc finger DNA-binding transcription proteins, which are involved in numerous pathophysiological processes, including cell differentiation, apoptosis and tumor formation (10–12). KLFs are closely associated with cardiovascular diseases, including hypertension, AS and coronary heart disease (10–12). KLF15 is a member of the zinc finger protein family (13). It has been previously demonstrated that KLF15 is expressed in the heart, liver, kidney and numerous other organs (14,15). Furthermore, KLF15 is involved in the pathological processes of nephropathy, abnormal glucose metabolism and myocardial injury (16,17). However, the function of KLF15 in vascular endothelial dysfunction remains unclear. Nuclear factor (NF)-κB is a transcription factor that is highly expressed in mammals and highly conserved among mammalian species (18). Initially, NF-κB was considered to be a homologous/heterogeneous dimer composed of Katanin p60 ATPase-containing subunit A1 and transcription factor p65 (p65) subunits (18); however, subsequent studies have revealed that there is an NF-κB protein family, which consists of several polypeptides with a high degree of homology (18,19). Abnormal activation of NF-κB may cause rheumatoid arthritis, AS, inflammation and tumor formation (20). Furthermore, previous studies have demonstrated that nuclear factor erythroid 2-related factor 2 (Nrf2) signaling may inhibit the activation of NF-κB during inflammation (21,22). In addition, NF-κB and Nrf2 always interact during oxidative stress and numerous inflammatory responses (23).
In the present study, the function of KLF15 in TNF-α-induced vascular endothelial dysfunction was investigated, in addition to whether its underlying molecular mechanisms are involved in the regulation of the NF-κB and Nrf2 signaling pathways.
Materials and methods
Cell culture and treatment
The human umbilical vein fusion cell line (Eahy926) was obtained from Shanghai Fuhengbio Biotechnology Co., Ltd. (Shanghai, China). Cells were maintained in RPMI-1640 (Beijing Hua Yueyang Biotechnology Co., Ltd., Beijing, China) supplemented with 10% fetal bovine serum (Jiangsu Enmoasai Biological Technology Co., Ltd., Changzhou, China) and 1% penicillin-streptomycin (Shanghai Yuanmu Biotechnology Co., Ltd., Shanghai, China), in a 37°C humidified incubator (MG80; Shanghai LNB Instruments Co., Ltd, Shanghai China) with 5% CO2. Following culture, Eahy926 cells were treated with PBS (Control) or different concentrations of TNF-α (1, 5, 10 and 20 ng/ml) in 37°C incubator for 48 h.
Cell transfection
Human pTA2-KLF15 (targeting sequence: 5′-ACAGAGACGTTGTGCTGCTTT-3′) and negative control pTA2 vectors were synthesized by Genewiz Biotechnology Co., Ltd. (Suzhou, China). Eahy926 cells were incubated in 6-well plates (2.5×104 cell/well) for 12 h at 37°C and, once 40–60% confluence had been reached, the cells were transfected with the aforementioned vectors (30 nM) using Lipofectamine™ 2000 transfection reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). After 48 h of transfection, cells were retained for subsequent experiments. Transfection efficiency was evaluated by RT-qPCR and western blot analysis.
MTT analysis
The viability of Eahy926 cells was investigated using an MTT kit (Gefan, Shanghai, China), according to the manufacturer's protocol. Firstly, Eahy926 cells were incubated in 96-well plates (2×103 cell/well) for 24 h and subsequently treated with 0.1% PBS (control group), pTA2 vector (NC group), pTA2-KLF15 vector (KLF15 group), 10 ng/ml TNF-α (TNF-α group), pTA2 vector and 10 ng/ml TNF-α (TNF-α + NC group), and pTA2-KLF15 vector and 10 ng/ml TNF-α (TNF-α + KLF15 group). Following this, 15 µl MTT solution was added to each well and the plates were subsequently incubated at 37°C for 6 h. Following this, 0.2% dimethyl sulfoxide was added to the cells and subsequently agitated using an for 15 sec at room temperature. Finally, the optical density (OD) values were determined at 450 nm using a microplate reader (cat. no. HBS-1096A; Nanjing Detie Experimental Equipment Co., Ltd., Nanjing, China).
NO analysis
The quantity of NO released by Eahy926 cells was determined using a NO detection kit (Beyotime Institute of Biotechnology, Shanghai, China), according to the manufacturer's protocol. Firstly, cells were digested using trypsin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), resuspended in RPMI-1640 medium, and the supernatant was subsequently added to 96-well plates. Following this, 5 µl NADPH, 10 µl flavine adenine dinucleotide (FAD) and 5 µl nitrate reductase was added to each well and subsequently incubated at room temperature for 40 min. Following this, LDH was added to the plates in a dropwise manner at 37°C for 18 min. Subsequently, cells were fixed using Griess reagent I/II at room temperature for 10 min. Finally, OD values were determined at 540 nm using a microplate reader.
Cell adhesion analysis
The adhesive ability of Eahy926 cells was investigated using a cell adhesion detection kit (BestBio Company, Shanghai, China), according to the manufacturer's protocol. Firstly, coating liquid (BestBio Company) was added into 96-well plates in a drop-wise manner. Following this, plates were transferred to a 4°C refrigerator (cat. no. BCD-216SDN; Qingdao Haier, Co., Ltd., Qingdao, China) for 24 h. Cells were then digested using trypsin, resuspended in RPMI-1640 medium and subsequently incubated in 96-well plates (2×103 cells/well) in a 37°C incubator for 30 min. Following this, 20 µl staining solution B (BestBio Company) was added to cells for 2 h. Finally, OD values were determined at 450 nm using a microplate reader, and cells were photographed under an inverted fluorescent microscope (×200; cat. no. GMSP-5; Guangmi, Shanghai, China). The adhesion rate was calculated using the following formula: Adhesion rate (%)=[(ODtest-ODblank)/(ODcontrol-ODblank)]x100.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated and lysed using an RNA extraction kit (BioTeke Corporation, Beijing, China). cDNA was subsequently synthesized using a RevertAid™ cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). The temperature protocol used for RT was as follows: 42°C for 60 min and 70°C for 5 min. Following this, qPCR was performed using a DreamTaq Green PCR master Mix kit (Thermo Fisher Scientific, Inc.), which included the following reagents: 25 µl Dream Taq Green PCR master Mix, 1 µl forward primer, 1 µl reverse primer, 19 µl nuclease-free distilled water, 4 µl cDNA, total volume 50 µl. The thermocycling conditions used for qPCR were as follows: Initial degeneration at 94°C for 5 min; followed by 30 cycles of denaturation at 94°C for 30 sec and annealing at 65°C for 30 sec; and a final extension at 72°C for 30 sec. Primer sequences used for qPCR are presented in Table I. β-actin was used as an internal control. The 2−ΔΔCq method was used to determine gene expression (24).
Table I.
Sequences of primers used for reverse transcription-quantitative polymerase chain reaction.
| Primer name | Sequence, 5′→3′ | Product size, bp |
|---|---|---|
| eNOS forward | GCTAGCCAAAGTCACCATCG | 230 |
| eNOS reverse | TGGAAAACAGGAGTGAGGCT | |
| KLF15 forward | TAGTCAACATCCAGGGGCAG | 212 |
| KLF15 reverse | GGAAACTTCTGGCCCACAAG | |
| MCP-1 forward | AGCCACCTTCATTCCCCAAG | 192 |
| MCP-1 reverse | GGGTCAGCACAGATCTCCTT | |
| ICAM-1 forward | CAGTCACCTATGGCAACGAC | 238 |
| ICAM-1 reverse | GCAGCTTACAGTGACAGAGC | |
| TGF-β1 forward | TACAGCAACAATTCCTGGCG | 200 |
| TGF-β1 reverse | GTGAACCCGTTGATGTCCA | |
| p65 forward | GAGGAGCACAGATACCACCA | 220 |
| p65 reverse | AGCCTCATAGAAGCCATCCC | |
| Nrf2 forward | TGAGCCCAGTATCAGCAACA | 171 |
| Nrf2 reverse | AGTGAAATGCCGGAGTCAGA | |
| β-actin forward | CACCATGTACCCAGGCATTG | 180 |
| β-actin reverse | TCGTACTCCTGCTTGCTGAT |
eNOS, endothelial nitric oxide synthase; KLF15, Krüppel-like factor 15; MCP-1, monocyte chemoattractant protein-1; ICAM-1, intercellular adhesion molecule-1; TGF-β1, transforming growth factor-β1; p65, transcription factor p65; Nrf2, nuclear factor erythroid 2-related factor 2.
Western blot analysis
Total proteins were isolated and lysed with radioimmunoprecipitation assay buffer (cat. no. 20101ES60; Shanghai Shengsheng Biotechnology Co., Ltd., Beijing, China), and the total protein concentration was determined using a Bradford protein detection kit (Yeasen, Beijing, China). Firstly, proteins (30 µg/lane) were separated via 12% SDS-PAGE analysis and then transferred to a polyvinylidene difluoride membrane. Subsequently, membranes were blocked with 5% non-fat milk at 37°C for 1 h. The membrane was then incubated with anti-KLF15 (cat. no. ab2647; 1:1,000; Abcam, Cambridge, UK), anti-endothelial nitric oxide synthase (eNOS; cat. no. AF950; 1:700; R&D Systems, Inc., Minneapolis, MN, USA), anti-monocyte chemoattractant protein-1 (MCP-1; cat. no. MAB679, 1:1,000; R&D Systems, Inc.), anti-intercellular adhesion molecule-1 (ICAM-1; cat. no. BBA3; 1:800; R&D Systems, Inc.), anti-transforming growth factor-β1 (TGF-β1; cat. no. MAB1835; 1:1,200; R&D Systems, Inc.), anti-phosphorylated (p)-p65 (cat. no. MAB7226; 1:1,000; R&D Systems, Inc.), anti-Nrf2 (cat. no. MAB3925; 1:1,000; R&D Systems, Inc.) and anti-β-actin (cat. no. MAB8969; 1:2,000; R&D Systems, Inc.) at 4°C overnight. Following hybridization, the primary antibodies were reclaimed and stored in a 4°C refrigerator, and membranes were subsequently washed with TBS containing 0.1% Tween-20. Following washing, membranes were incubated with the following corresponding secondary antibodies at 37°C for 60 min: Horseradish peroxidase (HRP)-tagged goat anti-mouse IgG H&L (cat. no. ab6789; 1:7,000; Abcam), HRP-tagged rabbit anti-mouse IgG H&L (cat. no. ab6728; 1:8,000; Abcam) and HRP-tagged rabbit anti-goat IgG H&L (cat. no. ab97100; 1:8,000; Abcam). Finally, the blots were visualized by enhanced chemiluminescent reagent (EMD Millipore, Billerica, MA, USA). Images were captured with a Fuji LAS-3000 imaging system (Fuji Photo Film Co., Ltd., Tokyo, Japan) and analyzed with ImageJ 1.48u software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data were expressed as the mean ± standard deviation using Microsoft Excel 2007 software (Microsoft Corporation, Redmond, WA, USA). A Student's t-test was used to compare the differences between two groups. Differences between multiple groups were determined using one-way analysis of variance followed by Tukey's post-hoc test. GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA) was used to draw the graphs. Experiments were performed in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
TNF-α induces dysfunction in Eahy926 cells
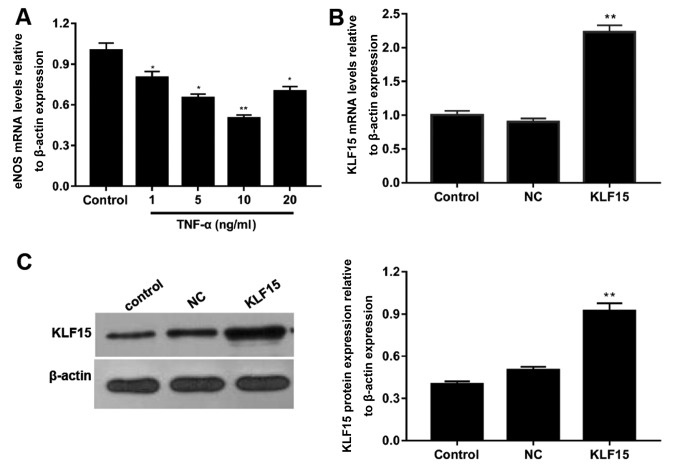
To determine the optimal concentration of TNF-α required to induce dysfunction in Eahy926 cells, cells were treated with various concentrations of TNF-α and the mRNA expression levels of eNOS were subsequently determined using RT-qPCR. Following treatment of cells with 1, 5, 10 and 20 ng/ml TNF-α, mRNA expression levels of eNOS were decreased by ~20, 35, 50 and 30% compared with the control group, respectively (Fig. 1A). Furthermore, the expression of eNOS was most significantly suppressed in cells treated with 10 ng/ml TNF-α (Fig. 1A; P<0.05).
Figure 1.
TNF-α induces endothelial dysfunction in Eahy926 cells. (A) Eahy926 cells were treated with 0.1% PBS (control) and TNF-α (1, 5, 10 and 20 ng/ml). mRNA expression levels of eNOS were investigated using RT-qPCR. *P<0.05 and **P<0.01 vs. control. KLF15 mRNA and protein levels in Eahy926 cells transfected with human pTA2-KLF15 and negative control pTA2 were investigated using (B) RT-qPCR and (C) western blot analyses, respectively. β-actin was used as an internal control. *P<0.05 and **P<0.01 vs. NC. NC, negative control pTA2 vector; KLF15, Krüppel-like factor 15; TNF-α, tumor necrosis factor-α; eNOS, endothelial nitric oxide synthase; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
To investigate the transfection efficiency of the pTA2-KLF15 vector, RT-qPCR and western blot analyses were performed to detect the mRNA and protein expression levels of KLF15, respectively. Following the transfection of pTA2-KLF15 vector into cells, the expression levels of KLF15 were revealed to be significantly enhanced in the KLF15 group compared with the NC and control groups (Fig. 1B and C; P<0.01). Furthermore, the expression levels of KLF15 in NC and control groups were revealed to be similar (Fig. 1B and C).
Overexpression of KLF15 attenuates the viability of TNF-α induced Eahy926 cells
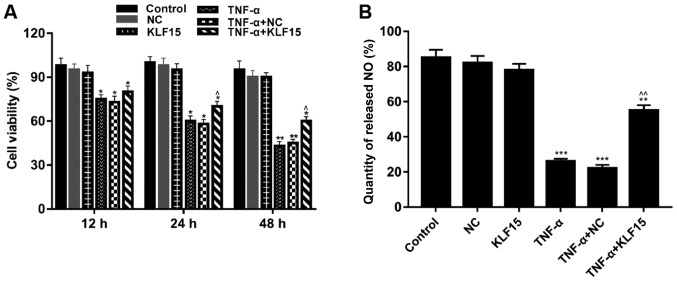
MTT assays were performed to investigate the viability of Eahy926 cells. The results demonstrated that treatment with TNF-α significantly suppressed the viability of cells compared with non-treated cells. (Fig. 2A; P<0.05 and P<0.01). Furthermore, when KLF15 was overexpressed in Eahy926 cells, the viability of Eahy926 cells did not exhibit a significant difference compared with the NC group; however, cell viability was significantly enhanced following overexpression of KLF15 in TNF-α-induced Eahy926 cells compared with the TNF-α + NC group. Cells overexpressing KLF15 at the 48 h time interval exhibited significantly suppressed cell viability, compared with the 12 h time interval (Fig. 2A; P<0.05).
Figure 2.
Overexpression of KLF15 attenuates the viability of Eahy926 cells treated with TNF-α. (A) Eahy926 cells were either treated with0.1% PBS (control), transfected with pTA2 vector, transfected with pTA2-KLF15 vector, treated with 10 ng/ml TNF-α, transfected with pTA2 vector and treated with 10 ng/ml TNF-α or transfected with pTA2-KLF15 vector and treated with 10 ng/ml TNF-α. The viability of Eahy926 cells was investigated using an MTT kit. (B) An NO detection kit was used to investigate the quantity of NO released by Eahy926 cells. *P<0.05, **P<0.01 and ***P<0.001 vs. respective NC. ^P<0.05, ^^P<0.01 and vs. respective TNF-α + NC. NC, negative control pTA2 vector; KLF15, Krüppel-like factor 15; TNF-α, tumor necrosis factor-α; NO, nitric oxide.
To investigate the quantity of NO released by Eahy926 cells, an NO detection kit was used. The results demonstrated that treatment with TNF-α significantly decreased the quantity of NO released by Eahy926 cells compared with the NC group (Fig. 2B; P<0.001). Furthermore, the results revealed that Eahy926 cells overexpressing KLF15 did not exhibit a significant difference regarding the quantity of released NO compared with the NC group (Fig. 2B). However, the quantity of released NO was significantly enhanced following overexpression of KLF15 in TNF-α-induced Eahy926 cells compared with the TNF-α + NC group (Fig. 2B; P<0.01).
Overexpression of KLF15 suppresses adhesion of TNF-α induced Eahy926 cells
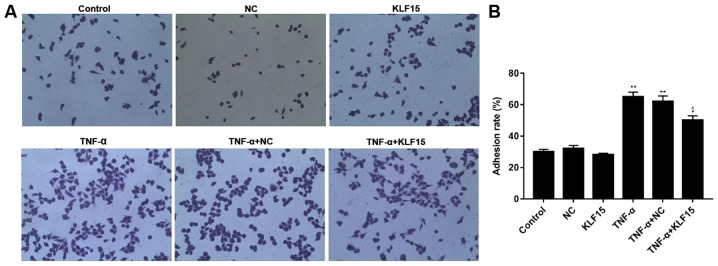
The rate of adhesion in Eahy926 cells was investigated using a cell adhesion detection kit. The results revealed that the rate of adhesion in Eahy926 cells overexpressing KLF15 did not exhibit a marked difference compared with the NC group, whereas TNF-α-induced Eahy926 cells exhibited a significantly increased adhesion rate compared with the NC group (Fig. 3; P<0.01). Furthermore, overexpression of KLF15 in TNF-α-induced Eahy926 cells was revealed to significantly suppress the rate of adhesion compared with the TNF-α + NC group (Fig. 3; P<0.05).
Figure 3.
Overexpression of KLF15 suppresses the adhesion of Eahy926 cells induced by TNF-α. (A) A cell adhesion detection kit was used to investigate the adhesion of Eahy926 cells. (B) The rate of cell adhesion was quantified using GraphPad Prism 7. Magnification, ×200. *P<0.05 and **P<0.01 vs. NC; ^P<0.05 vs. TNF-α + NC. NC, negative control pTA2 vector; KLF15, Krüppel-like factor 15; TNF-α, tumor necrosis factor-α.
Overexpression of KLF15 regulates the expression of factors associated with endothelial cells in Eahy926 cells
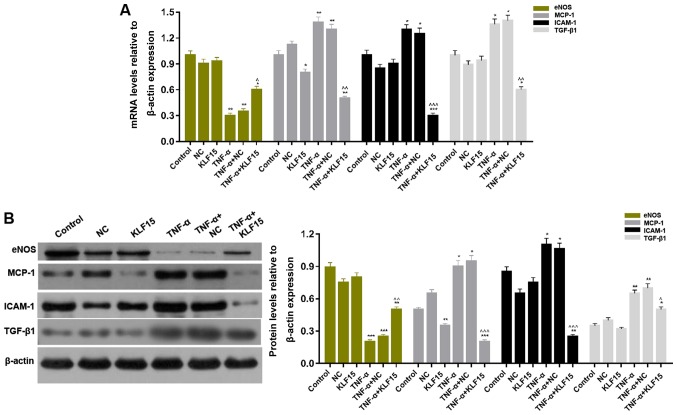
In order to determine the mRNA and protein expression levels of factors associated with endothelial cells in Eahy926 cells, RT-qPCR and western blot analyses were performed, respectively. The results demonstrated that treatment with TNF-α significantly upregulated them RNA and protein expression levels of MCP-1, ICAM-1 and TGF-β1 in Eahy926 cells; whereas, treatment with TNF-α significantly downregulated the mRNA and protein expression levels of eNOS in Eahy926 cells (Fig. 4; P<0.05 and P<0.01). Furthermore, the results revealed that overexpression of KLF15 in Eahy926 cells significantly decreased the levels of MCP-1 mRNA and protein; however, the mRNA and protein expression levels of eNOS, ICAM-1 and TGF-β1 in cells overexpressing KLF15 did not exhibit significant differences compared with the NC group. Furthermore, the mRNA and protein expression levels of MCP-1, ICAM-1 and TGF-β1 were significantly downregulated, and eNOS mRNA and protein expression levels were significantly upregulated, following overexpression of KLF15 in TNF-α-induced Eahy926 cells compared with the TNF-α + NC group (Fig. 4; P<0.05, P<0.01 and P<0.001).
Figure 4.
Overexpression of KLF15 regulates endothelial cell associated factors in Eahy926 cells. (A) mRNA expression levels of eNOS, MCP-1, ICAM-1 and TGF-β1 were determined via reverse transcription-quantitative polymerase chain reaction. (B) The protein expression levels of eNOS, MCP-1, ICAM-1 and TGF-β1 were investigated via western blotting. β-actin was used as an internal control. *P<0.05, **P<0.01 and ***P<0.001 vs. respective NC. ^P<0.05, ^^P<0.01 and ^^^P<0.001 vs. respective TNF-α + NC. NC, negative control pTA2 vector; KLF15, Krüppel-like factor 15; TNF-α, tumor necrosis factor-α; eNOS, endothelial nitric oxide synthase; MCP-1, monocyte chemoattractant protein-1; ICAM-1, intercellular adhesion molecule-1; TGF-β1, transforming growth factor-β1.
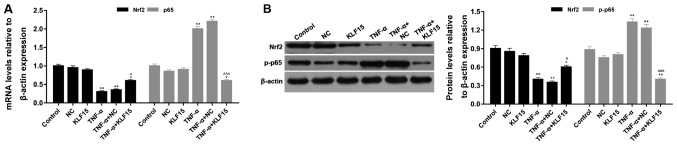
Overexpression of KLF15 regulates the NF-κB and Nrf2 signaling pathways in Eahy926 cells
The mRNA and protein expression levels of p65/phosphorylated (p)-p65 and Nrf2 were investigated using RT-qPCR and western blot analyses, respectively. The results of the RT-qPCR analyses demonstrated that treatment with TNF-α significantly suppressed Nrf2 mRNA expression and significantly enhanced p65 mRNA expression compared with the NC group (Fig. 5; P<0.01). Eahy926 cells overexpressing KLF15 did not exhibit any significant differences regarding the mRNA expression levels of p65 and Nrf2 compared with the NC group in the absence of TNF-α (Fig. 5). However, when Eahy926 cells overexpressed KLF15 were treated with TNF-α, the mRNA expression levels of Nrf2 were significantly increased, and p65 mRNA expression levels were significantly decreased, compared with the TNF-α + NC group (Fig. 5; P<0.05 and P<0.001). Furthermore, the results of the western blot analyses revealed that the expression trends of p-p65 and Nrf2 protein were similar to the expression trends of p65 and Nrf2 mRNA (Fig. 5; P<0.05, P<0.01 and P<0.001). These results suggested that overexpression of KLF15 regulated NF-κB and Nrf2 signaling pathways in Eahy926 cells.
Figure 5.
Overexpression of KLF15 regulates NF-κB and Nrf2 signaling pathways in Eahy926 cells. (A) Reverse transcription-quantitative polymerase chain reaction analysis was performed to investigate the mRNA expression of Nrf2 and p65. (B) Western blotting was performed to investigate the protein expression levels of Nrf2 and p-p65, which was normalized to β-actin expression. *P<0.05, **P<0.01 and ***P<0.001 vs. respective NC; ^P<0.05, ^^P<0.01 and ^^^P<0.001 vs. respective TNF-α + NC. NC, negative control pTA2 vector; KLF15, Krüppel-like factor 15; TNF-α, tumor necrosis factor-α; Nrf2, nuclear factor erythroid 2-related factor 2; p65, transcription factor p65; p-, phosphorylated.
Discussion
NO is the predominant factor released by endothelial cells, and it functions as a vasodilator that is able to attenuate endothelial dysfunction (25). Furthermore, NO functions as a intercellular signal and its synthesis is regulated by three factors: eNOS gene expression levels, eNOS activity and NO bioavailability (26). Thus, the function of endothelial cells may be investigated via determination of the quantity of released NO and the expression levels of eNOS. Furthermore, TNF-α is frequently utilized to induce various injuries in scientific research (27,28), and induces endothelial dysfunction in diabetic mouse hearts (29). The results of the present study demonstrated that eNOS mRNA expression levels in Eahy926 cells treated with 1, 5 and 10 ng/ml TNF-α decreased in a dose-dependent manner. However, when Eahy926 cells were treated with 20 ng/ml TNF-α, eNOS mRNA expression levels were upregulated compared with cells treated with 10 ng/ml TNF-α. It was hypothesized that self-repair mechanisms performed by injured endothelial cells may be associated with increased expression levels of eNOS. Therefore, treatment with 10 ng/ml TNF-α was chosen to induce Eahy926 cells for the construction of the vascular endothelial dysfunction model for subsequent experiments in the present study.
KLFs are involved in numerous pathological processes associated with cardiovascular disease and are associated with the regulation of various signal transduction pathways (30). KLF2 has been demonstrated to induce the expression of numerous bioactive factors that have a protective role in the cardiovascular system (31). In addition, it has been revealed that overexpression of KLF11 attenuates TNF-α-induced damage to human umbilical vein endothelial cells (HUVECs) (32). Therefore, it was hypothesized that KLF15 may have a role in the TNF-α-induced dysfunction of vascular endothelial cells. The results demonstrated that overexpression of KLF15 attenuated the viability of Eahy926 cells otherwise inhibited by TNF-α, enhanced the quantity of released NO and enhanced eNOS expression. These results suggested that overexpression of KLF15 attenuated TNF-α-induced dysfunction in Eahy926 cells.
Vascular endothelial injury may increase the adhesion rate of endothelial cells or platelets, and induce the formation of vasoactive molecules, including ICAM-1; chemotactic factors, including MCP-1; and growth factors, including TGF-β (33,34). Previous studies have revealed that KLF2 is able to regulate the secretion and release of inflammatory factors and adhesion factors, including ICAM-1, thereby inhibiting the adhesion of leukocytes, platelets and vascular endothelial cells (33,35). Furthermore, HUVECs overexpressing KLF11 have been demonstrated to exhibit suppressed expression levels of MCP-1 and ICAM-1 (32). Considering the results of the aforementioned studies, the present study aimed to investigate whether overexpression of KLF15 affected adhesive ability and the expression levels of MCP-1, ICAM-1 and TGF-β1 in Eahy926 cells. The results revealed that overexpression of KLF15 decreased the rate of adhesion and attenuated the expression levels of MCP-1, ICAM-1 and TGF-β1 in Eahy926 cells induced by TNF-α.
An increasing number of studies have suggested that activation of the NF-κB signaling pathway has an important role in endothelial dysfunction (36,37). The activation of NF-κB is closely associated with the upregulation of MCP-1 and ICAM-1 expression levels, which represent biomarkers of endothelial dysfunction (38,39). KLF11 is able to regulate the expression of downstream inflammatory and adhesion factors by binding top65 (32). Furthermore, previous studies have demonstrated that KLF2 is able to activate the expression of Nrf2, which subsequently stimulates the production of antioxidants in endothelial cells (40). Thus, we hypothesized that overexpression of KLF15 may have a protective effect on TNF-α-induced dysfunction in Eahy926 cells, which may regulate NF-κB and Nrf2 signaling pathways. The results of the present study revealed that Eahy926 cells overexpressed KLF15 exhibited activated Nrf2 signaling and inhibited NF-κB signaling, which attenuated TNF-α-induced dysfunction in endothelial cells.
In conclusion, the results of the present study revealed that TNF-α was able to induce vascular endothelial dysfunction in Eahy926 cells at an optimum concentration of 10 ng/ml. Furthermore, overexpression of KLF15 in Eahy926 cells was demonstrated to have a protective effect on the TNF-α-induced dysfunction of endothelial cells via activation of Nrf2 signaling and inhibition of NF-κB signaling. Therefore, KLF15 may represent a novel therapeutic target for the treatment of patients with atherosclerosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by Qingdao City South District Science and Technology Program (grant no. 2538), the Youth Fund of The Affiliated Hospital of Qingdao University (grant no. 2616) and the Qingdao University ‘Clinical Medicine +X’ Engineering Fund (grant no. 2017M24).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
BL, LLX, HFW and SX made substantial contributions to the conception and design of the study. XMY and MJG implemented the experiments. XMY, WL and XZS were responsible for analyzing and interpreting the data. BL and MJG drafted the manuscript. All authors were responsible for giving approval of the final version of the manuscript to be published.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Salzer L, Tenenbaum-Gavish K, Hod M. Metabolic disorder of pregnancy (understanding pathophysiology of diabetes and preeclampsia) Best Pract Res Clin Obstet Gynaecol. 2015;29:328–338. doi: 10.1016/j.bpobgyn.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Ding W, Cheng H, Chen F, Yan Y, Zhang M, Zhao X, Hou D, Mi J. Adipokines are associated with hypertension in Metabolically Healthy Obese (MHO) children and adolescents: A prospective population-based cohort study. J Epidemiol. 2018;28:19–26. doi: 10.2188/jea.JE20160141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30:569–572. doi: 10.1016/S1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 4.Kim TJ, Shin HY, Chang Y, Kang M, Jee J, Choi YH, Ahn HS, Ahn SH, Son HJ, Ryu S. Metabolically healthy obesity and the risk for subclinical atherosclerosis. Atherosclerosis. 2017;262:191–197. doi: 10.1016/j.atherosclerosis.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Moon S, Oh CM, Choi MK, Park YK, Chun S, Choi M, Yu JM, Yoo HJ. The influence of physical activity on risk of cardiovascular disease in people who are obese but metabolically healthy. PLoS One. 2017;12:e0185127. doi: 10.1371/journal.pone.0185127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caffarelli C, Montagnani A, Nuti R, Gonnelli S. Bisphosphonates, atherosclerosis and vascular calcification: Update and systematic review of clinical studies. Clin Interv Aging. 2017;12:1819–1828. doi: 10.2147/CIA.S138002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra R, O'Neil GL, Harding IC, Cheng MJ, Mensah SA, Ebong EE. Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr Atheroscler Rep. 2017;19:63. doi: 10.1007/s11883-017-0691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 10.Bieker JJ. Krüppel-like factors: Three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 11.Chiambaretta F, De Graeve F, Turet G, Marceau G, Gain P, Dastugue B, Rigal D, Sapin V. Cell and tissue specific expression of human Krüppel-like transcription factors in human ocular surface. Mol Vis. 2004;10:901–909. [PubMed] [Google Scholar]
- 12.Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN, Wu M. Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966–970. doi: 10.3748/wjg.v8.i6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Cai L. Does krüppel like factor 15 play an important role in the left ventricular hypertrophy of patients with type 2 diabetes? EBioMedicine. 2017;20:17–18. doi: 10.1016/j.ebiom.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shou F, Xu F, Li G, Zhao Z, Mao Y, Yang F, Wang H, Guo H. RASSF1A promoter methylation is associated with increased risk of thyroid cancer: A meta-analysis. Onco Targets Ther. 2017;10:247–257. doi: 10.2147/OTT.S124417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, et al. Role of Krüppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem. 2005;280:12867–12875. doi: 10.1074/jbc.M410515200. [DOI] [PubMed] [Google Scholar]
- 16.Leenders JJ, Wijnen WJ, van der Made I, Hiller M, Swinnen M, Vandendriessche T, Chuah M, Pinto YM, Creemers EE. Repression of cardiac hypertrophy by KLF15: Underlying mechanisms and therapeutic implications. PLoS One. 2012;7:e36754. doi: 10.1371/journal.pone.0036754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SK, Wai B, Lang CC, Levin D, Palmer CNA, Parry HM, Velkoska E, Harrap SB, Srivastava PM, Burrell LM. Genetic variation in kruppel like factor 15 is associated with left ventricular hypertrophy in patients with type 2 diabetes: Discovery and replication cohorts. EBioMedicine. 2017;18:171–178. doi: 10.1016/j.ebiom.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang C, Lin X. Analysis of epidermal growth factor-induced NF-κB signaling. Methods Mol Biol. 2015;1280:75–102. doi: 10.1007/978-1-4939-2422-6_6. [DOI] [PubMed] [Google Scholar]
- 19.Crofford LJ, Tan B, McCarthy CJ, Hla T. Involvement of nuclear factor kappa B in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum. 1997;40:226–236. doi: 10.1002/art.1780400207. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB. Nuclear factor-kappaB: The enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Cuadrado A, Martín-Moldes Z, Ye J, Lastres-Becker I. Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem. 2014;289:15244–15258. doi: 10.1074/jbc.M113.540633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JK, Lee JE, Jung EH, Jung JY, Jung DH, Ku SK, Cho IJ, Kim SC. Hemistepsin A ameliorates acute inflammation in macrophages via inhibition of nuclear factor-κB and activation of nuclear factor erythroid 2-related factor 2. Food Chem Toxicol. 2018;111:176–188. doi: 10.1016/j.fct.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Myers PR, Guerra R, Jr, Harrison DG. Release of NO and EDRF from cultured bovine aortic endothelial cells. Am J Physiol. 1989;256:H1030–H1037. doi: 10.1152/ajpheart.1989.256.4.H1030. [DOI] [PubMed] [Google Scholar]
- 26.Nathan C, Xie QW. Nitric oxide synthases: Roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 27.Pang Y, Zheng B, Fan LW, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55:1099–1107. doi: 10.1002/glia.20530. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Yang X, Wang H, Zhao B, Wu X, Su L, Xie S, Wang Y, Li J, Liu J, et al. PKCζ as a promising therapeutic target for TNFα-induced inflammatory disorders in chronic cutaneous wounds. Int J Mol Med. 2017;40:1335–1346. doi: 10.3892/ijmm.2017.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Lee S, Zhang H, Hill MA, Zhang C, Park Y. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One. 2017;12:e0187189. doi: 10.1371/journal.pone.0187189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang E, Nayak L, Jain MK. Krüppel-like factors in endothelial cell biology. Curr Opin Hematol. 2017;24:224–229. doi: 10.1097/MOH.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 31.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan Y, Guo Y, Zhang J, Subramaniam M, Song CZ, Urrutia R, Chen YE. Krüppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-κB signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32:2981–2988. doi: 10.1161/ATVBAHA.112.300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han JM, Li H, Cho MH, Baek SH, Lee CH, Park HY, Jeong TS. Soy-leaf extract exerts atheroprotective effects via modulation of krüppel-like factor 2 and adhesion molecules. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020373. pii: E373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain MK, Sangwung P, Hamik A. Regulation of an inflammatory disease: Krüppel-like factors and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:499–508. doi: 10.1161/ATVBAHA.113.301925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodiga VL, Kudle MR, Bodiga S. Silencing of PKC-α, TRPC1 or NF-κB expression attenuates cisplatin-induced ICAM-1 expression and endothelial dysfunction. Biochem Pharmacol. 2015;98:78–91. doi: 10.1016/j.bcp.2015.08.101. [DOI] [PubMed] [Google Scholar]
- 37.Tang X, Guo D, Lin C, Shi Z, Qian R, Fu W, Liu J, Li X, Fan L. hCLOCK causes Rho-kinase-mediated endothelial dysfunction and NF-κB-mediated inflammatory responses. Oxid Med Cell Longev. 2015;2015:671839. doi: 10.1155/2015/671839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell Mol Immunol. 2004;1:425–435. [PubMed] [Google Scholar]
- 39.Zhou Z, Connell MC, MacEwan DJ. TNFR1-induced NF-kappaB, but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells. Cell Signal. 2007;19:1238–1248. doi: 10.1016/j.cellsig.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Fledderus JO, Boon RA, Volger OL, Hurttila H, Ylä-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.