Abstract
Secukinumab, an interleukin (IL)-17A antagonist, was associated with disease exacerbations in Crohn’s disease, and de novo cases of inflammatory bowel disease (IBD) have been reported in studies of rheumatoid diseases. However, there has been no detailed report demonstrating the linkage between secukinumab therapy and new-onset IBD. We present a unique case of rapid-onset fulminant colitis after receiving 1 dose of secukinumab infusion followed by improvement with combination antibiotics, corticosteroids, and calcineurin inhibition. Health care providers should be aware of the possible association between IL-17 antagonist therapy and the risk of developing new-onset fulminant IBD.
Introduction
Interleukin (IL)-17 is a pro-inflammatory cytokine that has been demonstrated to be involved in the pathogenesis of inflammatory bowel disease (IBD).1,2 However, secukinumab, an IL-17A antagonist, has been associated with worsening symptoms compared to placebo in clinical trials of Crohn’s disease.3 Whether anti-IL 17 therapy is associated with new-onset fulminant IBD remains uncertain.
Case Report
A 41-year-old white woman with a history of psoriasis was admitted with severe abdominal pain, hourly bloody bowel movements, and temperature of 38.9°C. One month prior, she presented to an outside hospital with new-onset abdominal pain and hematochezia 1 week after receiving her first dose of secukinumab. Prior to the secukinumab treatment, she had tried adalimumab and ustekinumab, but had been off all immunosuppressive therapy for 3 months before starting secukinumab. This was the first time she had developed gastroenterological symptoms. She is a non-smoker. Her mother has a history of Crohn’s disease, and her daughter has ulcerative colitis. At the outside hospital, the patient was diagnosed with IBD unclassified and was discharged on 9 mg daily budesonide multi-matrix (MMX, Santarus, Inc., Raleigh, North Carolina). However, her symptoms continued to worsen, and she was referred to the IBD clinic at the University of Chicago.
On the current admission, she was initiated on intravenous methylprednisolone 40 mg/day. However, she developed a fever of 40.4°C with tachycardia. Her C-reactive protein (CRP) increased to 128 mg/L, albumin decreased from 3.5 to 3.1 mg/L, and hemoglobin decreased from 10.6 to 9.7 mg/L. Flexible sigmoidoscopy revealed diffuse moderate-to-severe colitis with friable mucosa and several deep ulcers in the proximal sigmoid colon (Figure 1). Histologic evaluation was consistent with moderately active IBD with erosions (Figure 2). Cytomegalovirus on biopsy was negative. Broad spectrum antibiotics (cefepime and metronidazole) were initiated, and cyclosporine 2 mg/kg continuous infusion was added as salvage therapy. Within 2 days, her fever resolved. She was discharged with CRP < 3 mg/L and was started on oral cyclosporine and a prednisone taper. At follow-up, she was clinically stable and was subsequently switched from cyclosporine to infliximab and methotrexate as maintenance therapy to simultaneously manage her IBD and psoriasis.
Figure 1.
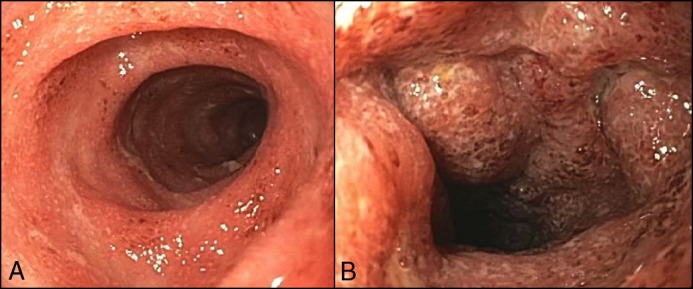
(A) Congested friable mucosa in the distal sigmoid colon. (B) Several deep ulcers and edema seen in the proximal sigmoid colon.
Figure 2.
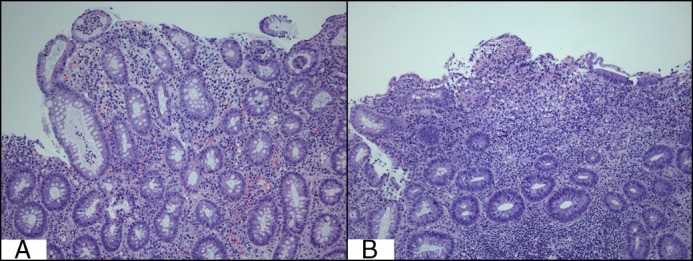
(A) Cryptitis seen on the biopsy of the sigmoid colon. (B) Erosions and severe infiltration of lymphoplasmacytic cells, consistent with chronic active colitis (20× magnification).
Discussion
This the first case report describing rapid onset of fulminant IBD after a single secukinumab infusion. We describe a rapid clinical response to the combination of antibiotics, corticosteroids, and calcineurin inhibition. Dysregulated immune response plays a critical role in the pathogenesis of IBD.1 IL-17 has been proposed as one of the pro-inflammatory cytokines involved in IBD.4,5 Increased mRNA concentration of IL-17A was found in the intestinal mucosa of patients with Crohn’s disease.6 Thus the blockade of IL-17 was thought to have therapeutic potential in treating IBD. However, several studies have reported increased adverse effects among IBD patients undergoing treatment with secukinumab. For example, secukinumab was ineffective in inducing remission of Crohn’s disease and was associated with higher rates of adverse events.3 This association was again demonstrated in the study of Crohn’s disease and brodalumab, which is a monoclonal antibody against the IL-17 receptor.7 The fact that blockade of either IL-17 ligand or its receptor has led to worsening symptoms of Crohn’s disease suggests that IL-17 may carry a protective effect in IBD.
Although IL-17 inhibition was associated with worsening symptoms in Crohn’s disease, it remains uncertain whether IL-17 blockade increases the risk of new-onset IBD. There have been a few incidents of IBD reported in clinical trials that investigated the usage of secukinumab in rheumatologic diseases. In the phase 3 trials of secukinumab in plaque psoriasis, 3 cases of ulcerative colitis and 1 case of Crohn’s disease were reported in the treatment groups versus none in the placebo groups during the 52-week treatment period.8 In the study of psoriatic arthritis, 1 case of Crohn’s disease and 1 case of ulcerative colitis were also reported, respectively, in the group treated with secukinumab.9 Although secukinumab may be associated with new-onset IBD on the basis of these reports, the risk appears low, with a total of 6 cases of new-onset IBD out of 1,758 patients who received secukinumab. Considering patients with psoriasis are at increased risk of developing IBD, these incidents may be due to chance alone.10
As a downstream molecule of IL-23, IL-17A is known to play a pro-inflammatory role in multiple human immune diseases.4 However, its role in IBD was recently challenged by several studies. In animal models, IL-17A was shown to be protective in T cell-mediated colitis, and IL-17A-deficient mice developed rapid weight loss and severe colitis.11,12 It has been postulated that loss of IL-17A could lead to an imbalance of inflammatory cytokines that favors the Th1 pathway and leads to development of severe colitis.13 In addition, defective genetic IL-17 immunity in humans was associated with a risk of chronic mucocutaneous candidiasis.14 A higher frequency of fungal infections were also reported in the patients with Crohn’s disease who were treated with secukinumab.3 Considering Candida could serve as an antigen for anti-Saccharomyces cerevisia (ASCA) antibodies, which is one of the most sensitive serological markers of Crohn’s disease, it is possible that inhibition of IL-17 could change the mycobiome composition in the gut that may predispose susceptible individuals to the development of IBD.15,16 Current evidence suggests that inhibition of IL-17 may induce inflammation in the gastrointestinal tract by favoring Th1 pathways and/or changing the mycobiome composition.
We describe the first case of new and rapid onset of fulminant IBD after a single infusion of secukinumab. The direct causal relationship is uncertain. Our patient might have developed IBD even without the infusion of secukinumab, considering her positive family history. However, clinicians should be aware of the possible association between IL-17 antagonist therapy and the risk of developing new-onset fulminant colitis, particularly in those with a strong family history of IBD.
Disclosures
Author contributions: J. Wang and A. Sakuraba wrote the manuscript. A. Bhatia, N. Krugliak Cleveland, N. Gupta, S. Dalal, and D. T. Rubin edited the manuscript. A. Sakuraba is the article guarantor.
Financial dislosure: S. Dalal received a research grant from Pfizer. D. T. Rubin receives grant support from Takeda, Janssen, and AbbVie, and is a consultant for Pfizer and Amgen. A. Sakuraba has conducted collaborative research with Abbvie and has received consultant fees from Abbvie and Celltrion.
Informed consent was obtained for this case report.
References
- 1.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–45. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T, Okamoto S, Hisamatsu T, et al. . IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–9. [DOI] [PubMed] [Google Scholar]
- 3.Hueber W, Sands BE, Lewitzky S, et al. . Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yen D, Cheung J, Scheerens H, et al. . IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujino S, Andoh A, Bamba S, et al. . Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targan SR, Feagan B, Vermeire S, et al. . A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn's disease. Am J Gastroenterol. 2016;111:1599–607. [DOI] [PubMed] [Google Scholar]
- 8.Langley RG, Elewski BE, Lebwohl M, et al. . Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- 9.Mease PJ, McInnes IB, Kirkham B, et al. . Secukinumab inhibition ofinterleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn's disease. J Eur Acad Dermatol Venereol. 2009;23:561–5. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor W Jr, Kamanaka M, Booth CJ, et al. . A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awasthi A, Kuchroo VK. IL-17A directly inhibits TH1 cells and thereby suppresses development of intestinal inflammation. Nat Immunol. 2009;10:568–70. [DOI] [PubMed] [Google Scholar]
- 14.Puel A, Cypowyj S, Bustamante J, et al. . Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulain D, Sendid B, Standaert-Vitse A, Fradin C, Jouault T, Jawhara S, Colombel JF. Yeasts: Neglected pathogens. Dig Dis. 2009;27(Suppl 1):104–10. [DOI] [PubMed] [Google Scholar]
- 16.Colombel JF, Sendid B, Jouault T, Poulain D. Secukinumab failure in Crohn's disease: The yeast connection? Gut. 2013;62:800–1. [DOI] [PubMed] [Google Scholar]


