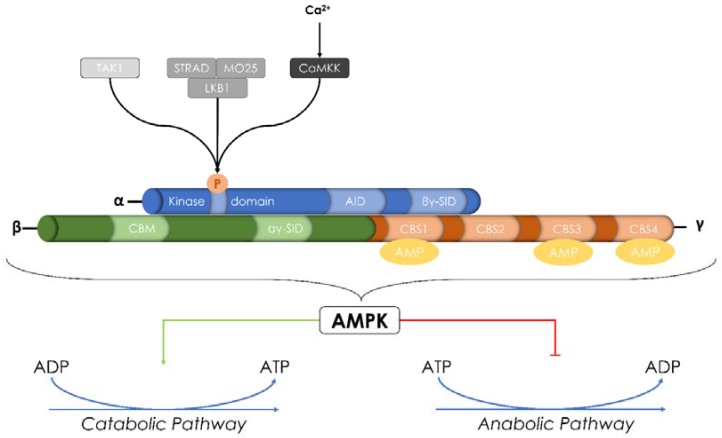
Figure 2.
Structure and activation of AMP-activated protein kinase (AMPK). AMPK is a heterotrimeric α-β-γ serine/threonine kinase. It is made up of a catalytic α-subunit complexed with regulatory β-and γ-subunits. It can be activated through the phosphorylation of Thr-172 by two main upstream kinases: Ca2+-Calmodulin Kinase Kinase (CaMKK) and Liver Kinase B1 (LKB1). Transforming growth factor-β-activated kinase (TAK1) was also described as a new AMPK regulatory kinase. In addition to its activation by upstream kinases, AMPK can also be allosterically activated by AMP. Once activated, AMPK responds to changes in the level of Adenosine triphosphate (ATP) by switching off either anabolic and biosynthetic pathways consuming ATP or switching on catabolic pathways that produce ATP.