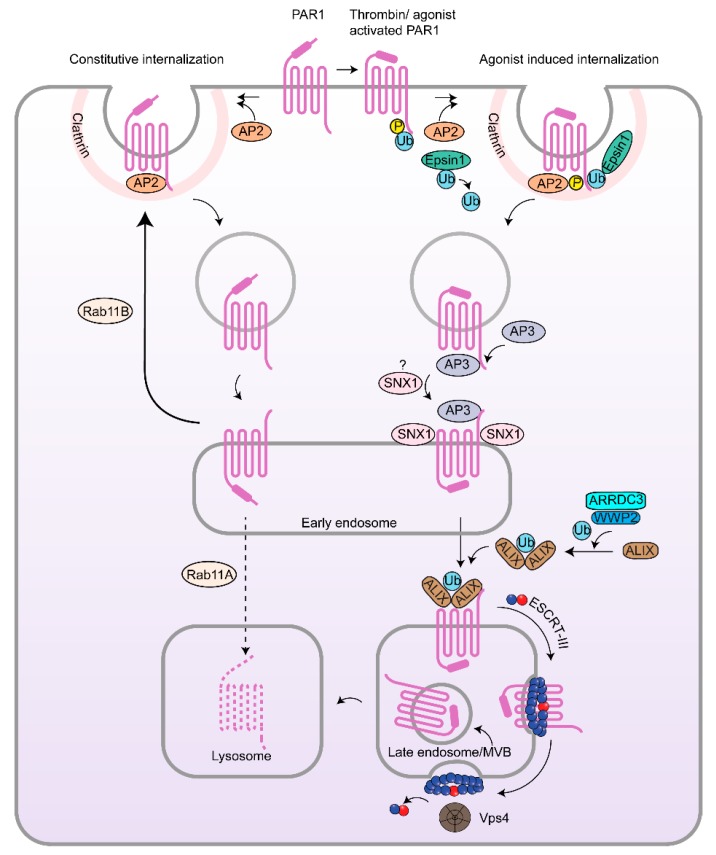
Figure 4.
Endocytic trafficking of PAR1. PAR1 undergoes constitutive and agonist-activated internalization induced by thrombin cleavage of the N-terminus or by the peptide agonist SFLLRN. Unactivated PAR1 is constitutively internalized by AP-2 recognition of a distal tyrosine-based motif within the cytoplasmic C-terminus of PAR1. Internalized PAR1 is then sorted to early endosomes and then recycled back to the cell surface via a Rab11B-dependent pathway, whereas a small pool of receptor escapes recycling and is sorted by Rab11A to lysosomes and degraded. In contrast, agonist activation of PAR1 results in rapid phosphorylation and ubiquitination and internalization through a dynamin- and clathrin-dependent pathway mediated by AP-2 and epsin-1. AP-2 binds the phosphorylated distal C-terminus of activated PAR1 rather than the tyrosine-based motif to regulate activated PAR1 internalization. PAR1 activation also promotes epsin-1 deubiquitination, facilitating the ability of epsin-1 to bind activated PAR1 to facilitate internalization. Internalized PAR1 is then sorted sequentially at early endosomes by engaging AP-3 and SNX1 followed by ALIX, which requires ARRDC3 and WWP2-mediated ALIX ubiquitination and dimerization. ALIX, ARRDC3 and WWP2 are essential for targeting PAR1 to intraluminal vesicles of multivesicular bodies (MVBs)/late endosomes via ECSRT-III charged MVB protein 4 (CHMP4) and AAA-ATPase vacuolar protein sorting 4 (Vps4). Degradation of PAR1 in lysosomes is ultimately required for signal termination.