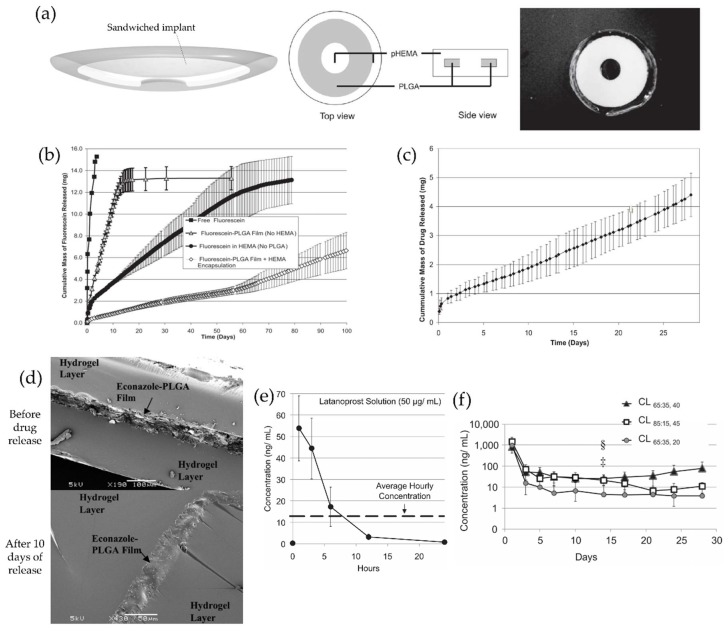
Figure 4.
(a) Schematic of a therapeutic contact lens embedded with sandwiched polymeric implant (left, middle) and photograph (right) of a ciprofloxacin-loaded PLGA implant-embedded poly-HEMA-contact lens with a 5-mm clear central zone; (b) Cumulative drug release profiles for 100 days from free fluorescein powder, fluorescein-PLGA films, fluorescein-coated poly-HEMA-contact lens and poly-HEMA-contact lens embedded with fluorescein-PLGA films; (c) Ciprofloxacin release from PLGA implant-embedded contact lenses in vitro. Reprinted with permission from [63]; (d) SEM images (X190) of econazole-PLGA films within prototype contact lenses made of poly-HEMA hydrogel in before drug release (up) and in after 10 days of release (down). Reprinted with permission from [64]; (e) Concentration of latanoprost in aqueous humor after topical instillation of latanoprost solution (50 μg/mL); (f) Concentrations of latanoprost in aqueous humor in rabbits wearing latanoprost-eluting contact lenses. Reprinted with permission from [65].