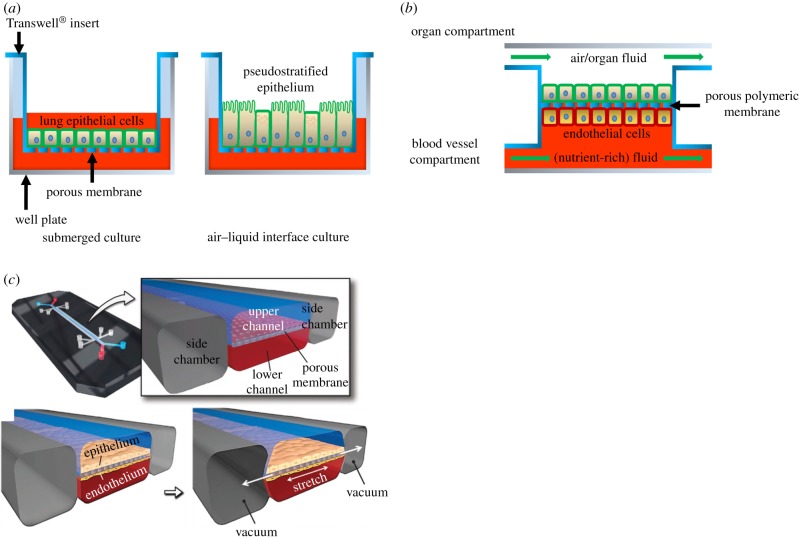
Figure 1.
Schematic representation of lung cell culture systems. (a) Lung epithelial cells are cultured in a conventional manner on a porous poly(bisphenol-A carbonate) (PC) or poly(ethylene terephthalate) (PET) membrane using a commercially available Transwell® system. Initially, cell culture medium is provided both on top of the cells and below the insert, i.e. submerged culture. Subsequently, the medium on top of the cells is removed to expose the cells to air, i.e. air–liquid interface culture. This causes the cells to transition to a pseudostratified epithelium. Nutrients from the cell culture medium pass through the membrane. (b) OOCs generally comprise two compartments that are connected via a porous, polymeric membrane. The blood vessel compartment contains a flowing fluid which substitutes the blood in the particular organ, often containing the nutrients and other factors which the cells need, such as cell culture medium. Endothelial cells can be cultured on the membrane to represent the blood vessel wall. The organ compartment holds cells of the organ of interest, for example liver, kidney or lung epithelial cells which are grown on the other side of the membrane. The compartment is usually filled with a medium mimicking the fluid in the organ or, for example, air, in the case of the lung or skin. In most OOCs, this medium flows to mimic the flow of air or liquid in the organ. (c) A lung-on-a-chip consisting of a microfluidic device with three channels. The middle channel contains two compartments, separated by a porous poly(dimethyl siloxane) (PDMS) membrane. Lung epithelial cells are cultured on the top side, while endothelial cells are cultured on the bottom side of the membrane. Air and cell culture medium flow through the top and bottom compartment, respectively. A vacuum can be applied in the adjacent two channels which provide mechanical stretch to the membrane and cells. Adapted from Huh et al. [1]. (Online version in colour.)