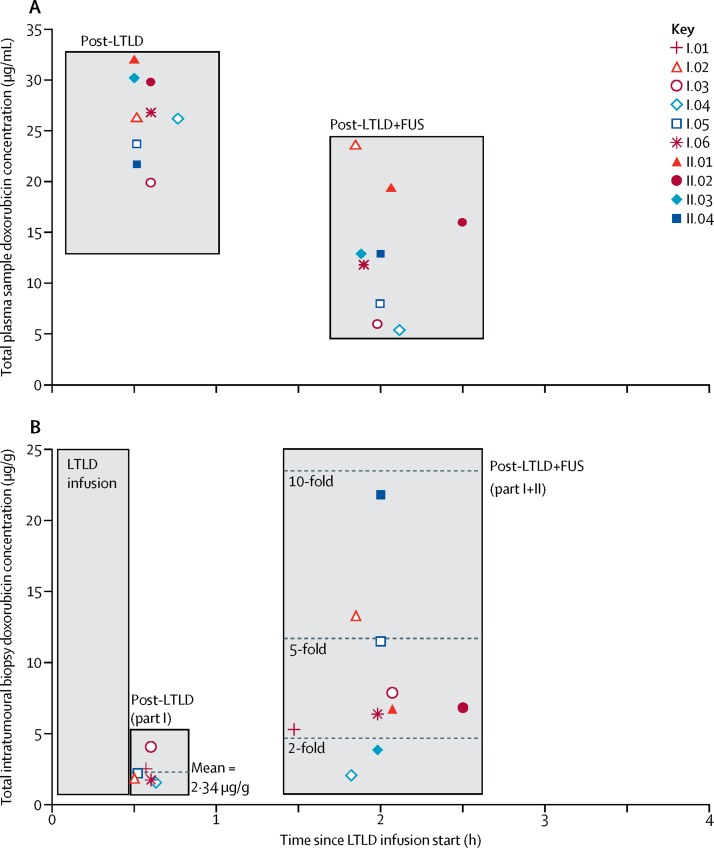
Figure 2.
Total doxorubicin concentration in plasma and tumour samples analysed by high-performance liquid chromatography (HPLC)
(A) Lyso-thermosensitive liposomal doxorubicin (LTLD) plasma pharmacokinetic data by HPLC. Data for patient I.01 are omitted from the plot, because concentrations were much greater than the top standard, resulting in a ten-fold dilution step for plasma analysis subsequently being introduced to the assay. (B) Intratumoural pharmacokinetic data by HPLC. The post-LTLD values for patient I.02 and I.06, and the post-LTLD plus focused ultrasound (FUS) values for patient I.06 are worst-case estimates.