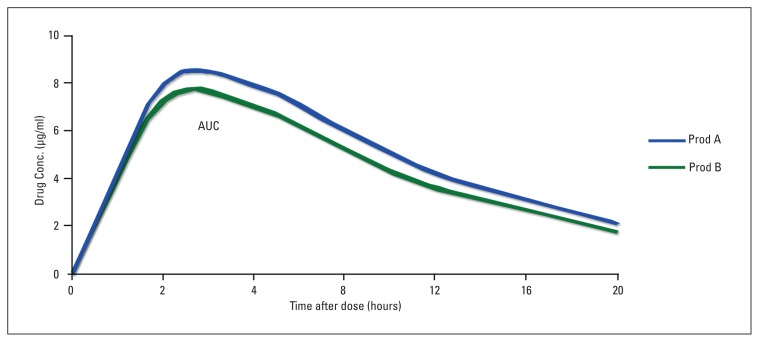
Figure 1.
Bioequivalence of a generic product (B) and a reference product (A). Product A is the reference product. Product B is the test (generic) product. The relevant parameters are: Drug A: Cmax=8.1 μg/ml; Tmax=2.6 h; AUCo-∞=124.9 μg.h/ml Drug B: Cmax=7.6 μg/ml; Tmax=2.1 h; AUCo-∞=112.4 μg.h/ml The ratio of areas (generic:reference), and therefore the relative bioavailability, is 0.9 To be accepted as bioequivalent, the 90% Confidence Intervals for the area ratio would need to fall within the 0.8–1.25 range* Adopted from Ref 3.