Figure 1.
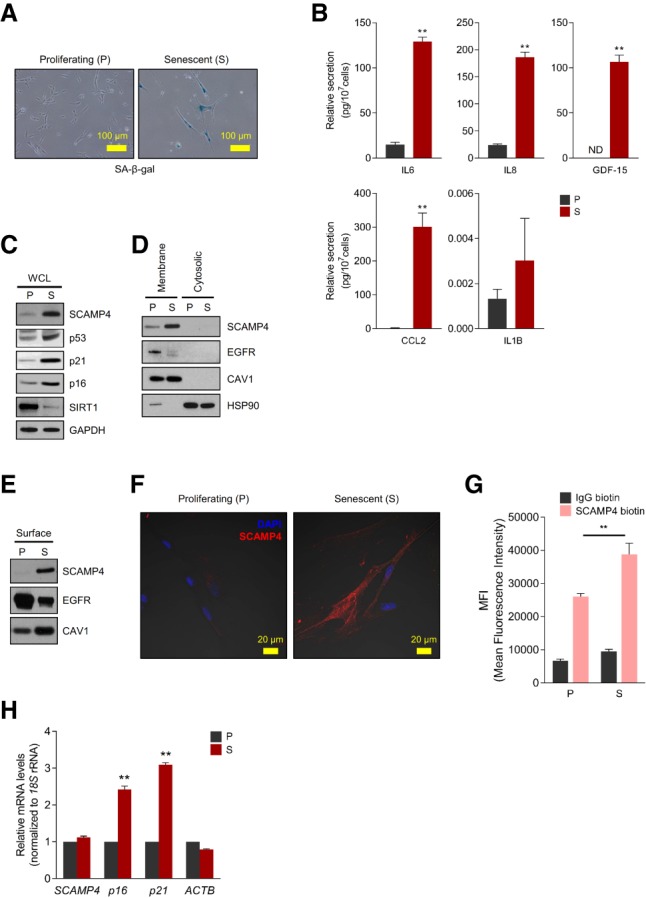
Identification of SCAMP4 as a cell surface protein selectively elevated in senescent WI-38 fibroblasts. (A) SA-β-gal analysis of proliferating (P; PDL 20) and senescent (S; PDL 50) WI-38 human diploid fibroblasts. (B) ELISA measurement of secreted IL6, IL8, GDF-15, CCL2, and IL1B in conditioned media from proliferating and senescent WI-38 cells, normalized to cell number. (ND) Not detectable. (C–E) Western blot analysis of SCAMP4 levels in whole-cell lysate (WCL) (C), membrane and cytosolic lysates (D), and cell surface (E) from proliferating and senescent WI-38 fibroblasts. Senescence markers SIRT1, p53, p16, and p21; cytosolic marker HSP90; plasma membrane protein markers CAV1 (Caveolin-1) and EGFR; and loading control GAPDH were also assayed. (F) Proliferating and senescent WI-38 cells were fixed with 100% methanol, and endogenous SCAMP4 protein was detected by confocal microscopy. Merged signals. (Blue) Nuclei stained with DAPI; (red) SCAMP4. (G) Detached WI-38 (proliferating and senescent) cells were incubated with anti-SCAMP4-biotin or control human IgG-biotin antibodies and then incubated with streptavidin-APC antibodies. SCAMP4-positive cells were analyzed by flow cytometry; mean fluorescence intensity (MFI) of SCAMP4-APC in proliferating and senescent cells was quantified. (H) Steady-state SCAMP4 mRNA levels were measured by reverse transcription followed by quantitative PCR (RT-qPCR) analysis. p16 and p21 mRNAs were included as senescent cell markers, and ACTB mRNA was included as a loading control; mRNA levels were normalized to 18S rRNA levels in each sample; mRNAs in proliferating cells were set as 1. Graphs in B, G, and H represent the means ± SEM from three independent experiments; (**) P-value < 0.01.
