Figure 4.
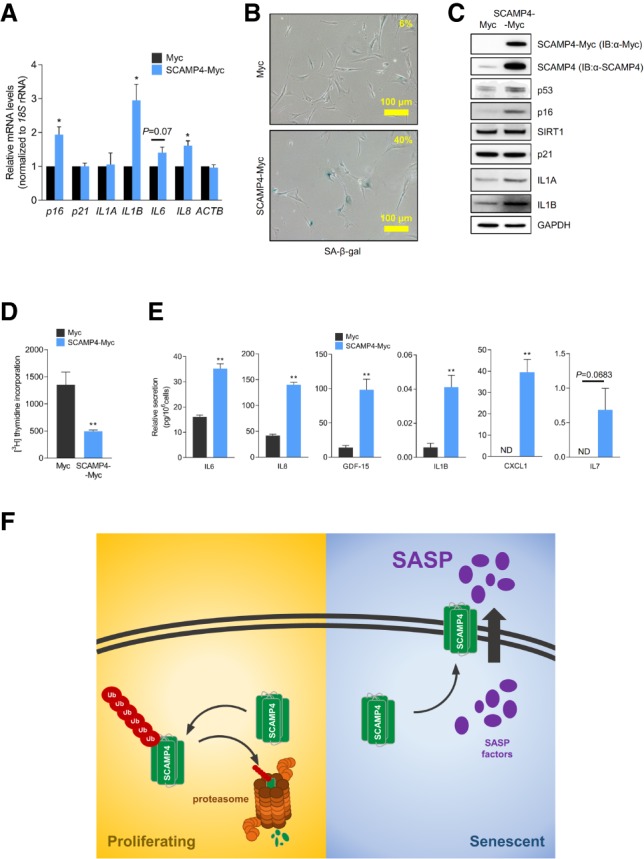
SCAMP4 promotes secretion of SASP factors. (A–E) Proliferating WI-38 cells were transduced with lentiviral particles to express either Myc tag alone or SCAMP4-Myc. Twenty days later, RT-qPCR analysis was used to measure the steady-state levels of p16, p21, IL1A, IL1B, IL6, IL8, and ACTB mRNAs (normalized to 18S rRNA levels) (A); SA-β-gal activity was assessed (B); Western blot analysis was performed to assess the levels of SCAMP4-Myc (using anti-SCAMP4 antibody or anti-Myc antibody), p53, IL1A, IL1B, p21, p16, SIRT1, and GAPDH (loading control) in whole-cell lysates (C); [3H]-thymidine incorporation was measured (D); and the secretion of IL6, IL8, GDF-15, IL1B, CXCL1, and IL7 was measured by ELISA (E). Graphs in A, D, and E represent the means ± SEM from three independent experiments. (**) P-value < 0.01; (*) P-value < 0.05. (F) Proposed model. In proliferating cells, SCAMP4 is rapidly degraded by the ubiquitin–proteasome degradation system. In senescent cells, SCAMP4 is stable and localizes to the cell surface, in turn promoting the secretion of SASP factors during senescence.
