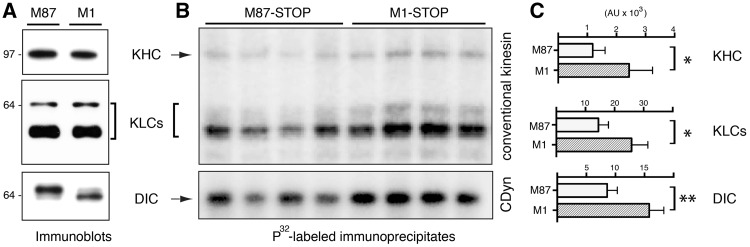
Figure 4.
Increased phosphorylation of motor proteins in association with M1-STOP expression. Stably-transfected SH-SY5Y cells expressing M1-STOP and M87-STOP were metabolically labeled using radiolabeled 32P. Lysates derived from these cells were processed for immunoprecipitation using antibodies that selectively immunoprecipitate the multi-subunit motor proteins conventional kinesin and cytoplasmic dynein (CDyn). (A)) Immunoblot analysis confirmed similar expression levels of KHCs and KLCs subunits of conventional kinesin, as well as DIC subunits of cytoplasmic dynein (CDyn) between M1-STOP (M1) and M87-STOP (M87) cells. (B) Autoradiogram analysis of 32P-labeled conventional kinesin and CDyn immunoprecipitates revealed increased phosphorylation of kinesin heavy (KHC) and light chain (KLCs) subunits, as well as dynein intermediate chain (DIC) subunits in cells expressing M1-STOP, compared to M87-STOP cells. (C) Phospho-imager-based quantitation of data in B. *P < 0.05; **P < 0.01.