Summary
Gastric neuroendocrine neoplasms (GNENs) are classified into three types according to their aetiology. We present a clinical case of a female patient of 66 years and a well-differentiated (grade 2), type 3 GNEN with late liver metastasis (LM). The patient underwent surgical excision of a gastric lesion at 50 years of age, without any type of follow-up. Sixteen years later, she was found to have a neuroendocrine tumour (NET) metastatic to the liver. The histological review of the gastric lesion previously removed confirmed that it was a NET measuring 8 mm, pT1NxMx (Ki67 = 4%). 68Ga-DOTANOC PET/CT reported two LM and a possible pancreatic tumour/gastric adenopathy. Biopsies of the lesion were repeatedly inconclusive. She had a high chromogranin A, normal gastrin levels and negative anti-parietal cell and intrinsic factor antibodies, which is suggestive of type 3 GNEN. She underwent total gastrectomy and liver segmentectomies (segment IV and VII) with proven metastasis in two perigastric lymph nodes and both with hepatic lesions (Ki67 = 5%), yet no evidence of local recurrence. A 68Ga-DOTANOC PET/CT was performed 3 months after surgery, showing no tumour lesions and normalisation of CgA. Two years after surgery, the patient had no evidence of disease. This case illustrates a rare situation, being a type 3, well-differentiated (grade 2) GNEN, with late LM. Despite this, it was possible to perform surgery with curative intent, which is crucial in these cases, as systemic therapies have limited efficacy. We emphasise the need for extended follow-up in these patients.
Learning points:
GNENs have a very heterogeneous biological behaviour.
Clinical distinction between the three types of GNEN is essential to plan the correct management strategy.
LMs are rare and more common in type 3 and grade 3 GNEN.
Adequate follow-up is crucial for detection of disease recurrence.
Curative intent surgery is the optimal therapy for patients with limited and resectable LM, especially in well-differentiated tumours (grade 1 and 2).
Background
Gastric neuroendocrine neoplasms (GNENs) represent about 7% of all digestive NET, with a prevalence rate of 35/100 000 (1, 2). According to the 2010 World Health Organization classification, they may present as neuroendocrine tumours (NETs) with Ki67 ≤2% (Grade 1 – G1), 3–20% (Grade 2 – G2) or neuroendocrine carcinoma (NEC) with Ki67 >20% (Grade G3) (1).
GNENs may be classified into three types, according to their aetiology: type 1 is associated with autoimmune atrophic gastritis, and it has the lowest metastatic potential from all three types; type 2 is histologically similar to type 1 but is associated with gastrinomas (Zollinger–Ellison syndrome), usually as part of MEN1 syndrome; type 3 is sporadic, without hypergastrinaemia or a gastric condition as predisponent factors. Type 3 lesions are larger and often accompanied by lymph node and distant metastases, resulting in a poor prognosis (1, 2, 3, 4, 5). Serum gastrin levels are crucial on determining the GNEN subgroup, i.e. type 3 GNEN is non-gastrin dependent, unlike type 1 and 2 GNEN. It is essential to obtain an adequate clinical, biochemical and pathological assessment of the tumour in order to plan the correct management strategy, as each of the 3 GNEN types have distinct biological behaviour and prognosis (2, 3).
The most common NEN with LM are pancreatic and small bowel NET. There is sparse data on the prevalence of LM in GNEN, although it is estimated that it rarely occurs in these patients and they are mostly synchronous (Table 1). At the time of the LM diagnosis, only 20–30% may still have the chance for curative intent surgery (5).
Table 1.
Classification of GNEN (1).
| GNEN | Type 1 | Type 2 | Type 3 |
|---|---|---|---|
| Prevalence (%) | 70–80 | 5–6 | 14–23 |
| Characteristics | 1–2 cm; multiple lesions | Isolated lesion (>2 cm); ulcerated | |
| Grade | G1 | G1–2 | G3 |
| Associated condition | Autoimmune atrophic gastritis | Gastrinoma/MEN 1 | – |
| Gastrin levels | Elevated | Elevated | Normal |
| Gastric pH | Elevated | Low | Normal |
| Metastases (%) | 2–5 | 10–30 | 50–100 |
| Mortality (%) | 0–1 | <10 | 25–30 |
Well-differentiated, type 3 GNEN are rare, as only two other cases are reported in medical literature (6). Also, liver involvement is unusual in GNEN patients and only 25% develop during disease course, which was the case for this patient. LM are more common in NETs with higher proliferation index (grade 3) and in type 3 GNEN, but this patient had a grade 2 lesion, which might explain the relatively indolent course of the disease (7, 8). In addition, the patient was able to have curative intent surgery, which is relatively uncommon in cases of NETs with LM metastases.
Case presentation
A 66-year-old female patient complained of epigastralgy, nausea and anorexia for 3 months. An abdominal ultrasound and CT were performed, which reported two hepatic lesions in segments IV (3.5 cm) and VII (2 cm) and a lesion between the pancreas and the stomach (4.3 cm). Liver biopsy revealed LM of a grade 2 NET (Ki67 = 10%). She was then sent to our department for further investigation and treatment.
Investigation
Anamnesis revealed that she had a gastric lesion excised 16 years previously but she did not know the histological result. Previous medical records showed that the gastric lesion was a NET and the histological review confirmed that the primary tumour was indeed a NET measuring 8 mm, pT1NxMx (Ki67 = 4%).
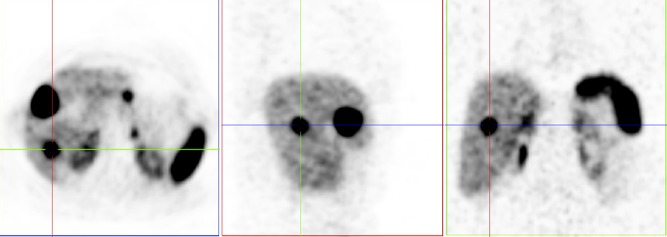
68Ga-DOTANOC PET/CT reported two liver metastases and a lesion between the stomach and pancreas, which was thought to be a possible pancreatic tumour/gastric adenopathy (Fig. 1). Biopsies of the lesion were repeatedly inconclusive. She had a high chromogranin A (CgA – 5685 ng/mL – normal range: <102 ng/mL), with no signs of pernicious anaemia (haemoglobin of 13.1 g/dL; normal mean corpuscular volume and mean corpuscular haemoglobin concentration). She also had normal gastrin levels and negative anti-parietal cell antibodies and anti-intrinsic factor antibodies. A gastric mapping was performed and the histological report on the gastric biopsies showed no signs of glandular atrophy or areas of intestinal metaplasia.
Figure 1.
68Ga-DOTANOC PET/CT reported two liver metastases and a lesion between the stomach and pancreas, which was thought to be a possible pancreatic tumour/gastric adenopathy.
Treatment
As the liver lesions were thought to be metastasis from a GNEN excised 16 years before and the perigastric lesion was thought to be a lymph node metastasis, the patient underwent total gastrectomy and liver segmentectomies (segment IV and VII). Histological report on the LM confirmed metastasis in two perigastric lymph nodes and both hepatic lesions (Ki67 = 5%), with positive staining for chromogranin and synaptophysin. The histological report on the stomach revealed mild glandular atrophy, but no areas of intestinal metaplasia or inflammation of the lamina propria and no evidence of local recurrence.
Outcome and follow-up
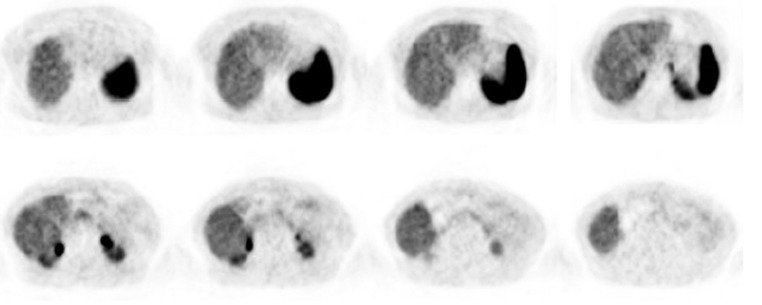
A 68Ga-DOTANOC PET/CT was performed 3 months after surgery, showing that the patient had no tumour lesions (Fig. 2). Thoracic-abdominal-pelvic CT scan done every 6 months showed no signs of disease recurrence and CgA levels are within normal range (69.6 ng/mL).
Figure 2.
68Ga-DOTANOC PET/CT performed 3 months after surgery showed no tumour lesions.
Discussion
GNENs have a very heterogeneous biological behaviour. Type 1 and 2 GNENs have a relatively benign clinical course, and the treatment strategy is focused on lesion resection and endoscopic follow-up. On the other hand, type 3 is usually associated with a higher proliferative index (grade 3), and therefore, a higher metastatic rate (50–100%). It may mimic the course of gastric adenocarcinoma and their treatments plans are identical (1). It is usually a solitary lesion, sporadic, and it is often associated with pain, anorexia and weight loss, as was the case with this patient.
This case was classified as a type 3 sporadic GNEN, as the patient had no hypergastrinaemia or autoimmune atrophic gastritis as predisponent factors. Despite the histological report on the stomach revealing mild glandular atrophy (but no areas of intestinal metaplasia), the patient showed no signs of pernicious anaemia, along with normal gastrin levels and negative anti-parietal cell antibodies and anti-intrinsic factor antibodies.
Furthermore, this is an uncommon situation because, despite being a sporadic GNEN, which would imply a worst outcome, it was classified as well-differentiated grade 2 GNEN. In addition, liver involvement is rarely observed in patients with GNENs and the majority of these cases have a high Ki67 and only 25% develop during disease course (7, 8). This is a very unusual case and only two other cases of well-differentiated type 3 GNENs are reported in medical literature (6). In one of the cases, the patient had a 10 cm GNEN (Ki67 = 7%), with no metastasis on diagnosis. On the other case, the patient had a 4 cm GNEN (Ki67 = 7%) with bilateral synchronous ovarian metastasis. Both cases highlight the biological heterogeneity of NETs, as they had the same Ki67 index, but different outcomes regarding disease dissemination.
The management of NET LM depends on its distribution. According to the ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms, there are three distinct patterns of liver infiltration by metastases and each one has a specific therapeutic approach: (i) liver metastases confined to one liver lobe or limited to two adjacent segments that can be resected by a standard anatomical resection (20–25% of the cases); (ii) LM found in one lobe primarily but with smaller satellites contralaterally (10–15% of the cases) and can still be handled surgically, including ablative approaches and (iii) diffuse, multifocal liver metastases (60–70% of the cases) that should not be treated surgically (9).
In this case, the two LMs were not located in adjacent segments, but the multidisciplinary team considered that this patient still had limited and resectable tumour burden. Therefore, the team recommended surgical resection. For patients with LM who are ineligible for complete resection, there are a variety of locally ablative procedures such as thermal ablative techniques, transarterial embolisation or chemoembolisation and radioembolisation. Other medical therapies include peptide receptor radionuclide therapy, somatostatin analogues and chemotherapy. However, there is very few evidence regarding the effectiveness of systemic treatment in patients with well-differentiated, type 3 GNEN. Therefore, surgery with curative intent is the most important treatment option in these cases and is associated with better long-term outcomes (9, 10). Alongside tumour grade, LM represents one of the most powerful predictors of survival (3, 4, 5, 7). Despite having a high likelihood of disease recurrence, the multidisciplinary team decided not to pursue any further adjuvant treatments, given that there is no evidence regarding its effectiveness. For that reason, it was decided to keep an active surveillance strategy, with clinical, imagiological and biochemical follow-up. The patient has been seen regularly and shows, thus far, no evidence of disease recurrence.
An adequate follow-up would perhaps be able to make a timely diagnosis of disease recurrence and enable the possibility of limited surgery. Type 3 GNENs have a higher risk of disease recurrence, and this should have prompted a prolonged follow-up. However, this is an uncommon case and there is no current evidence on which type of follow-up plan should be performed.
For biochemical follow-up, using chromogranin A as our tumour marker would be useful. Despite being relatively unspecific, it would have helped detect early disease recurrence as it was high when the patient was diagnosed with LM, and it normalised after liver surgery. Gastric endoscopy is regarded as a useful examination for follow-up of GNEN, but in this case, it did not detect disease recurrence. Endoscopic ultrasound, however, may have detected gastric adenopathies. Imaging examinations, such as thoracic-abdominal and pelvic CT, are crucial for the follow-up of cases like this one, with considerable risk of recurrence. On the other hand, given that this is a grade 2 tumour, with an indolent clinical course, the time span between imaging exams is controversial. A 68Ga-DOTANOC PET/CT would probably be recommended due to its high sensitivity regarding the diagnosis of metastasis and would complement this case, if any of the other imaging tests suggested the possibility of recurrence. After a few years beyond surgery, abdominal CT might be performed instead, as disease recurrence is more common in the liver and abdominal lymph nodes.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this case report.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
A written informed consent was obtained from the patient for publication of the submitted article and accompanying images.
Author contribution statement
Dr Bernardo Marques conducted the literature review and manuscript construction. Dr Raquel G Martins is the patient's main physician. Dr Guilherme Tralhão was the surgeon responsible for the surgical treatment. The other co-authors are part of the multidisciplinary tumour board who provided clinical care for the patient and were responsible for manuscript review.
References
- 1.Delle Fave G, O’Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, et al ENETS Consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology 2016. 103 119–124. ( 10.1159/000443168) [DOI] [PubMed] [Google Scholar]
- 2.Lepage C, Bouvier AM, Faivre J. Endocrine tumours: epidemiology of malignant digestive neuroendocrine tumours. European Journal of Endocrinology 2013. 168 77–83. ( 10.1530/EJE-12-0418) [DOI] [PubMed] [Google Scholar]
- 3.Li T-T, Qian Z-R, Wan J, Qi X-K, Wu B-Y. Classification, clinicopathologic features and treatment of gastric neuroendocrine tumors. World Journal of Gastroenterology 2014. 20 118–125. ( 10.3748/wjg.v20.i1.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel D, Chan D, Cehic G, Pavlakis N, Price TJ. Systemic therapies for advanced gastroenteropancreatic neuroendocrine tumors. Expert Review of Endocrinology and Metabolism 2016. 11 311–327. ( 10.1080/17446651.2016.1199952) [DOI] [PubMed] [Google Scholar]
- 5.Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases: treatment of neuroendocrine liver metastases. Cancer 2015. 121 1172–1186. ( 10.1002/cncr.28760) [DOI] [PubMed] [Google Scholar]
- 6.Manneh R, Castellano D, Caso O, Loinaz C, Jiménez J, Estenoz J, Calatayud M, Sepúlveda JM, García-Carbonero R. Well-differentiated grade 2, type 3 gastrointestinal neuroendocrine tumour with bilateral metastatic ovarian involvement: report of an unusual case. Case Reports in Oncology 2016. 9 255–261. ( 10.1159/000445940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey J-N, Rashid A, et al One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35 825 cases in the United States. Journal of Clinical Oncology 2008. 26 3063–3072. ( 10.1200/JCO.2007.15.4377) [DOI] [PubMed] [Google Scholar]
- 8.Grozinsky-Glasberg S, Thomas D, Strosberg J, Pape U, Felder S, Tsolakis A, Alexandraki K, Fraenkel M, Saiegh L, Reissman P, et al Metastatic type 1 gastric carcinoid: a real threat or just a myth? World Journal of Gastroenterology 2013. 19 8687–8695. ( 10.3748/wjg.v19.i46.8687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frilling A, Sotiropoulos GC, Li J, Kornasiewicz O, Plöckinger U. Multimodal management of neuroendocrine liver metastases HPB 2010. 12 361–379. ( 10.1111/j.1477-2574.2010.00175.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R. ENETS Consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012. 95 157–176. ( 10.1159/000335597) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a