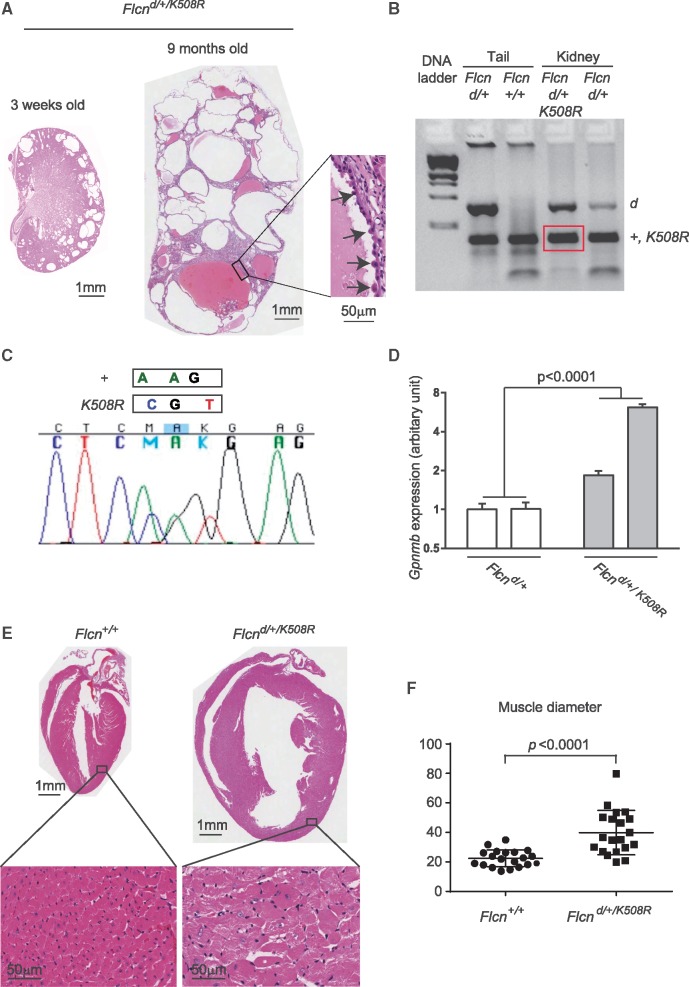
Figure 6.
Expression of BAC transgene carrying Flcn K508R mutant alleles in heterozygous Flcn knockout mice drives a late onset Flcn-deficient phenotype. (A) Histology of polycystic kidneys that developed in a portion of heterozygous Flcn knockout mice with Flcn K508R mutant expression (Flcn d/+/K508R) at 3 weeks and 9 months of age, respectively. Insert shows hyperplastic cells protruding into the cystic lumen.(B) PCR-based genotyping of DNA from macrodissected FFPE Flcn d/+/K508R cystic kidney tissue generated PCR products of the correct size for the Flcn deleted allele and for the Flcn wild-type allele and/or Flcn K508R transgene (both are the same size). DNA from macrodissected FFPE Flcn d/+ kidneys was genotyped and included as a control along with PCR-based genotyping of mouse tail DNA for comparison. +, wild-type allele; d, delete allele; Kid, kidney tissue. (C) Sequence verification of both wild-type Flcn and Flcn K508R transgene in PCR product from (B) amplified from DNA extracted from macrodissected Flcn d/+/K508R cystic kidneys. +, wild-type allele. (D) Gpnmb expression (read-out of Flcn deficiency) measured by qRT-PCR was statistically significantly higher in Flcn d/+/K508R kidneys compared to Flcn d/+ mouse kidneys. Statistical significance measured by two-way ANOVA. Two representative mouse kidneys for each genotype are shown. P <0.05 for significance. (E) Histology of cardiac hypertrophy that developed in a portion of heterozygous Flcn knockout mice with Flcn K508R mutant expression at 9 months of age. (F) Muscle diameter analysis of (E).