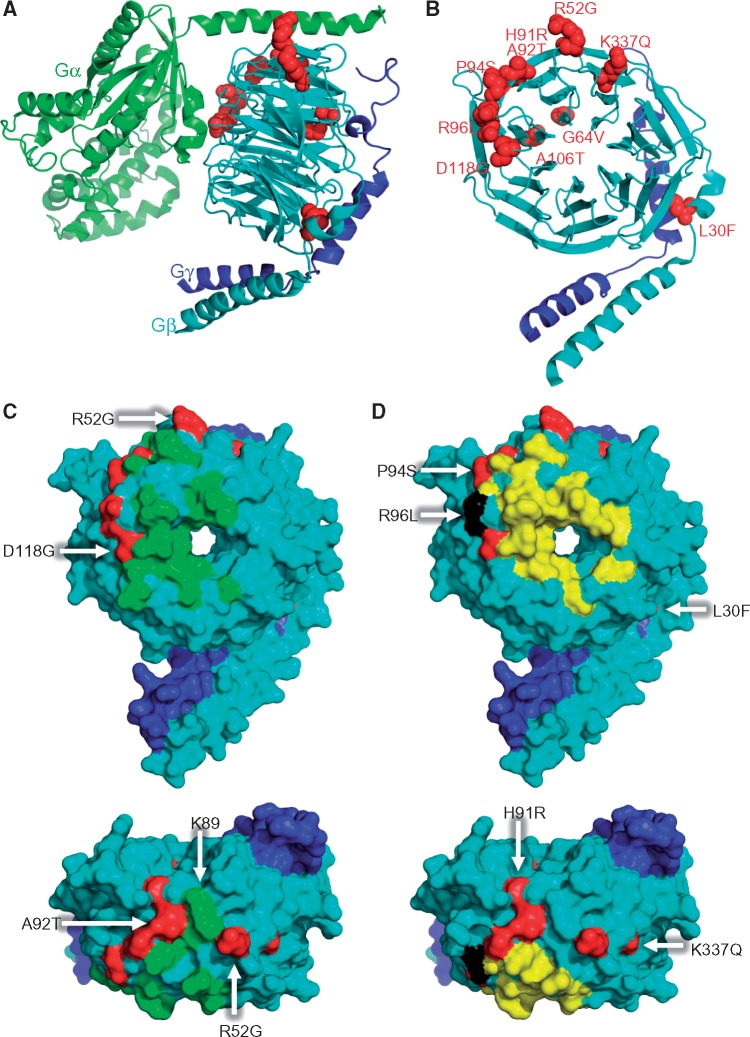
Figure 3.
Mutant residues of Gβ1 on three-dimensional crystal structures. (A) Cartoon representation of crystal structure of the Gαβγ trimer complex with mutations shown in red spheres. Gα, Gβ and Gγ are shown in green, cyan, and blue, respectively. The published crystal structure of Gαi1/Gβ1γ2 (PDB ID: 1GP2) was chosen as a model of the trimer (16). (B) Mutant residues in Gβ1γ2 dimer found in dystonia patients are highlighted and labeled by red spheres. (C, D) Binding sites on the molecular surface of Gα (C, green) and GRK (D, yellow) and on Gβ1 (cyan), respectively. Mutations are indicated in red. GRK2-Gβ1γ2 complex (PDB ID: 1OMW) (15) and Gαi1β1γ2 (PDB ID: 1GP2) were used to obtain the footprints. The residue colored in black indicates the overlap residue of the GRK binding site and the R96L mutation. The bird´s eye perspectives are shown at the bottom panels.