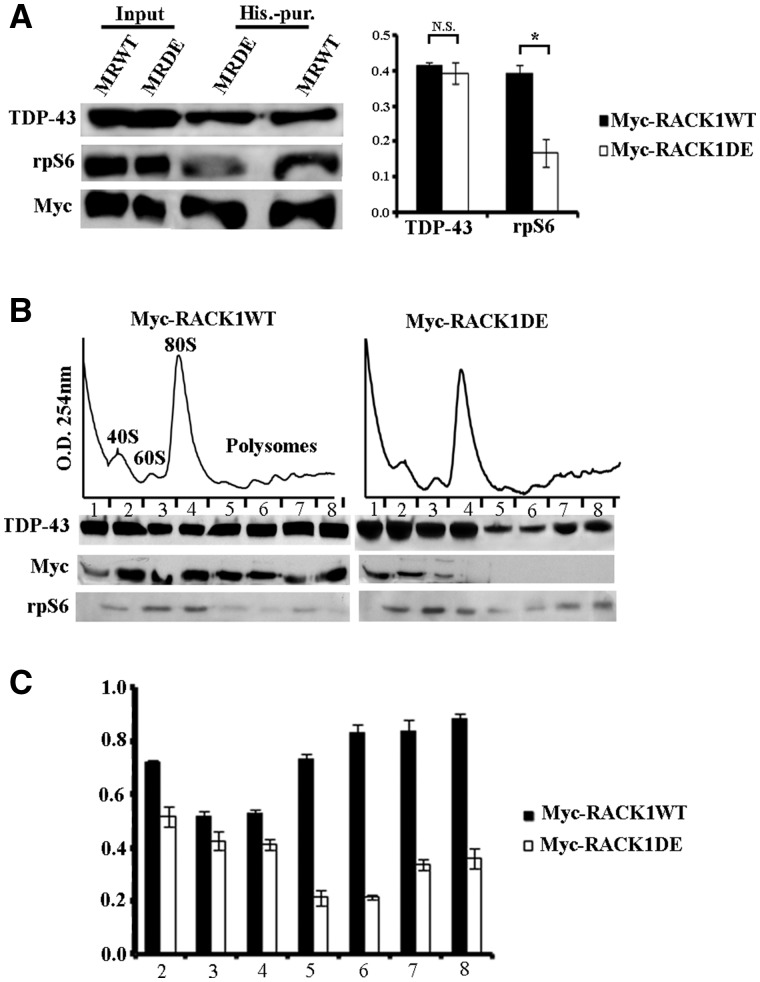
Figure 3.
TDP-43 associates the translational machinery by the binding to RACK1. (A) Left, immunoblotting for TDP-43, rpS6 and Myc antibodies on proteins eluted by histidine purification from SH-SY5Y cells overxpressing Myc-RACK1WT (MRWT) and Myc-RACK1DE (MRDE) proteins. The amount of TDP-43 purified from MRWT and MRDE imidazole eluted proteins was similar, while the level of rpS6 was reduced in purified MRDE. Right, quantification of TDP-43 and rpS6 co-purified with MRWT or MRDE measured by densitometry on bands related to immunoblottings of three independent experiments. All bar graphs represent the mean and Standard Deviation (S.D.) of TDP-43 or rpS6 level normalized to amount of purified Myc-fusion proteins. Student’s t-test was used to calculate P-values *<0.01. (B) Amount of TDP-43, examined by immunoblotting, on ribosomal fractions collected by polysome profiling conducted on SH-SY5Y cells overexpressing MRWT or MRDE proteins. The localization of TDP-43 on translational machinery was reduced by the overexpression of MRDE. The graphic in C quantifies amount of TDP-43 normalized to level of rpS6 on ribosome and polyribosome fractions (from 2 to 8 fractions) measured by densitometry on bands related to immunoblottings of three independent experiments. All bar graphs represent the mean and S.D.