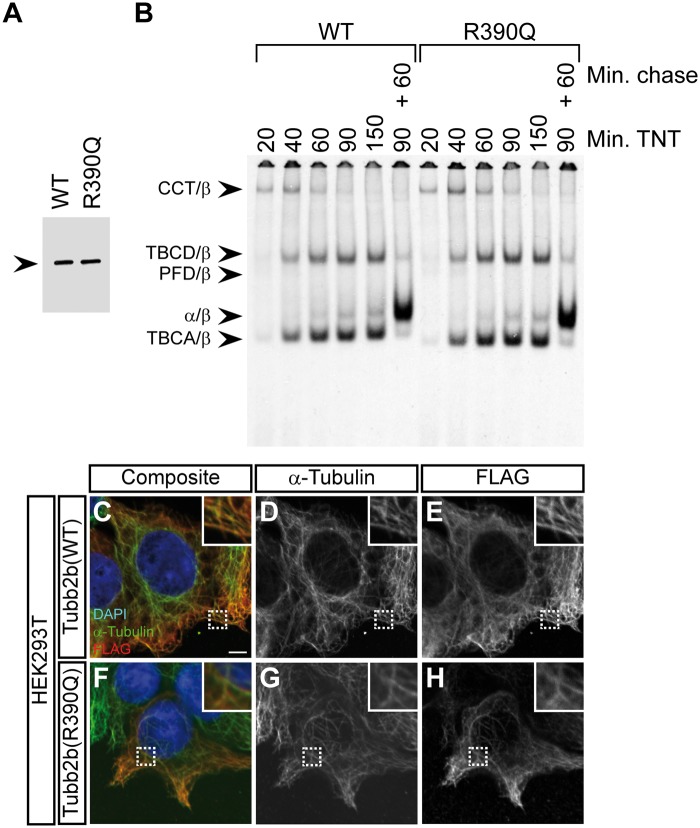
Figure 3.
The R390Q mutation does not affect tubulin folding or heterodimer integration into the microtubule lattice (A) Denaturing gel of in vitro transcribed and translated reaction products for wild-type (WT) and the TUBB2B R390Q mutant, in which the 35S-methinonine labelled proteins were detected by autoradiography. Note that the translational efficiency is not affected by the mutation. Arrowhead indicates the β-tubulin band. (B) Kinetic analysis of tubulin folding on non-denaturing gels of wild-type and R390Q translation products. Arrowheads (from top to bottom) denote the following complexes: chaperonin (CCT)/β-tubulin binary complex, the TBCD/β-tubulin co-complex, the prefoldin (PFD)/β-tubulin complex, the native tubulin heterodimer and the TBCA/β-tubulin co-complex. The molecular identities are assigned on the basis of their characteristic electrophoretic mobility. Shown are lanes for different reaction times (20, 40, 60, 90 and 150 minutes) in the rabbit reticulocyte lysate (TNT) and a combination of 90 minutes TNT and 60 minutes of chase with unlabelled wild-type tubulin. The R390Q mutation does not affect the kinetics of tubulin heterodimer formation or endpoint yields. (C–H) Immunofluorescence images of HEK293T cells transfected with FLAG-tagged wild-type Tubb2b (C–E) or mutant R390Q (F–H) and stained with antibodies against α-tubulin and the FLAG-tag. Shown are composite images (C and F) and grey-scale images of the individual channels (D, E, G and H). Wild-type and mutant tubulin heterodimers are both capable of integration into the microtubule lattice. Scale bar in (C) shows 5 µm. Magnifications of the dashed rectangles are shown in the upper right corner of each image.