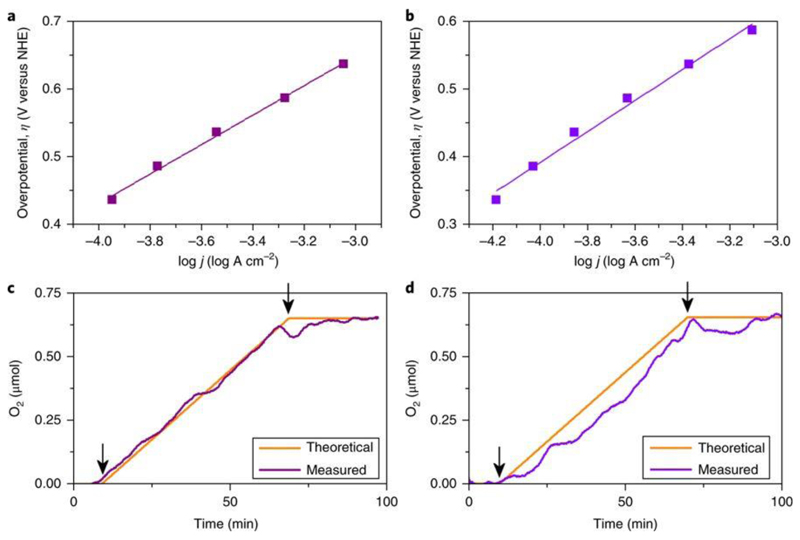
Figure 3. Electrochemical kinetics analysis of the compounds.
a,b, Tafel data for 3a[Cs] (a) and 3b[Cs] (b) extracted from doing 1 min bulk electrolysis scanning from 0.8 V to 1.4 V versus Ag/AgCl in 50 mV increments. pH 7.1, 50 mM KPi buffer, 1 M KNO3. c,d, Oxygen evolution during bulk water electrolysis with carbon paste electrodes containing 20% weight of compounds 3a[Cs] (c) and 3b[Cs] (d) performed at pH 7.1 in 50 mM KPi buffer, 1 M KNO3 at 1.4 V versus Ag/AgCl. Theoretical oxygen evolution was calculated estimating Faradaic production from current data (arrows indicate initial and final electrolysis times).