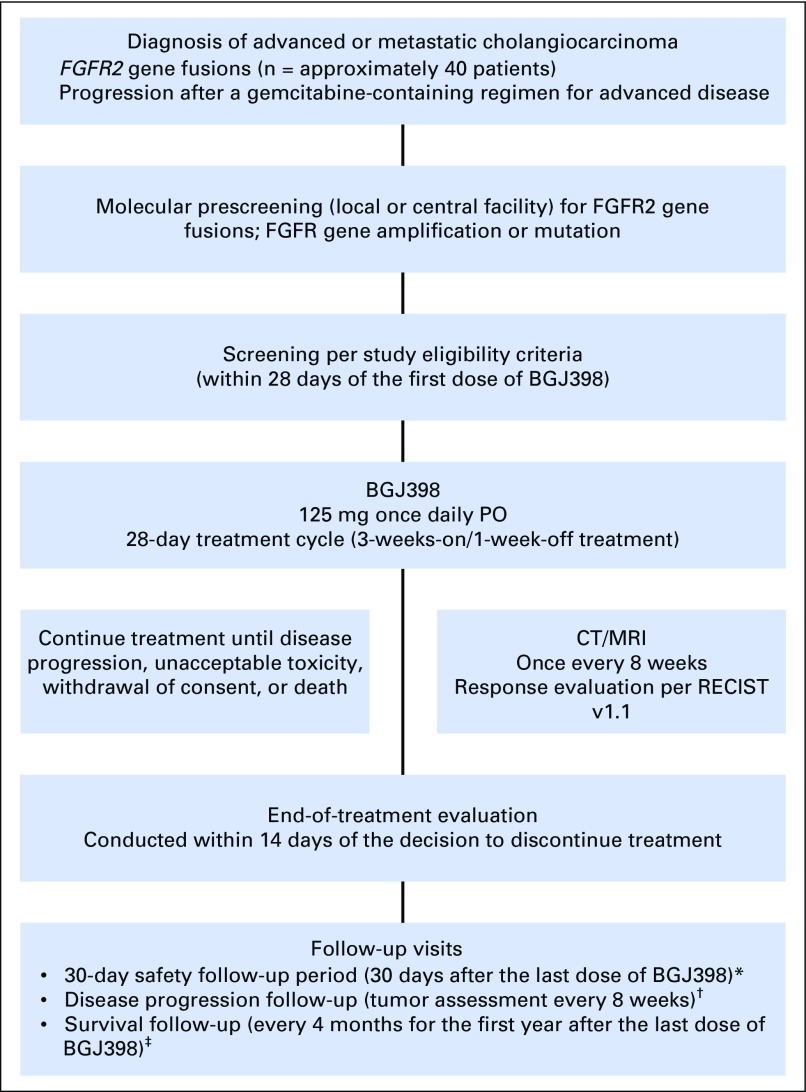
Fig A1.
Study design. (*) Safety follow-up was conducted for all patients. (†) Disease progression follow-up was conducted for patients who discontinued treatment for any reason other than disease progression. Follow-up continued until the first incidence of disease progression, initiation of a subsequent cancer therapy, or death. (‡) Survival follow-up will continue until all patients have discontinued study drug treatment and at least 80% of patients have died, withdrawn consent, or been lost to follow-up. CT, computed tomography; MRI, magnetic resonance imaging; PO, orally; RECIST, Response Evaluation Criteria in Solid Tumors.