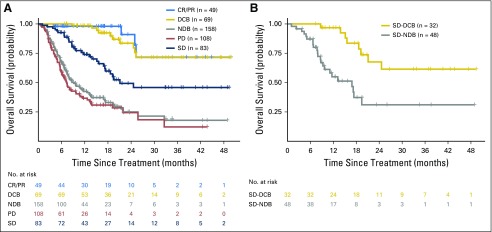
Fig A2.
Durable clinical benefit (DCB)/no durable benefit (NDB) compared with Response Evaluation Criteria in Solid Tumors (RECIST)–defined benefit. DCB and NDB are clinically useful, simple, binary outcomes to categorize those who benefit or not from immunotherapy. These groups have survival outcomes similar to RECIST-defined complete response (CR)/partial response (PR) or progressive disease (PD) while also incorporating meaningful distinction of those with stable disease (SD) who are benefiters or not. (A) Overall survival of patients with DCB/NDB or CR/PR, SD, or PD. Survival of DCB closely mirrors that of CR/PR, and NDB mirrors patients with PD. (B) A focus just on patients with SD shows a significant difference in overall survival stratified by DCB and NDB (P < .001). RECIST-defined SD, therefore, is an intermediate group that is comprised by a “true” benefit (progression-free survival [PFS] > 6 months) and not a “true” benefit (PFS < 6 months). Therefore, dichotomizing outcomes by duration of benefit more explicitly captures the major contribution of benefit from immunotherapy (durability), removes patients with uncommon short-lived responses, and improves adjudication of those with RECIST-defined SD, a heterogeneous group that comprises true benefit or not of immunotherapy.