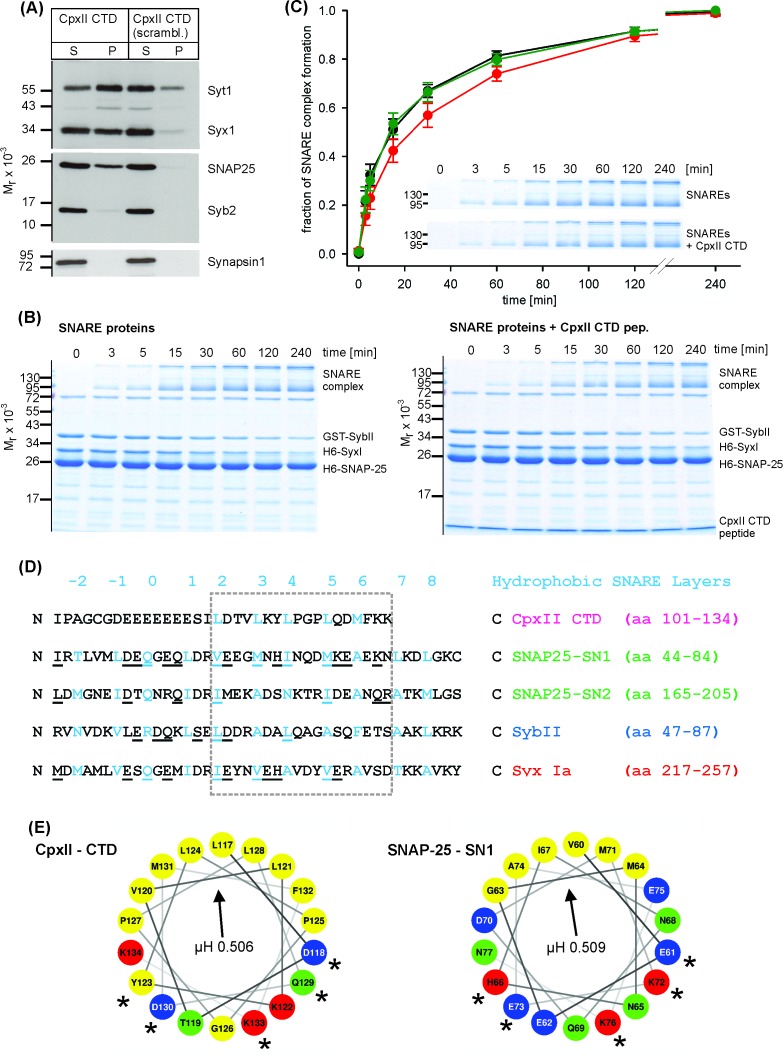
Figure 4. The CpxII CTD slows down the rate of SNARE complex formation.
(A) The CpxII CTD peptide co-precipitates SytI and preferentially the t-SNAREs, Syx1a and SNAP25, from detergent extract of mouse brain. Equal volumes of supernatant (S) and pellets (P) were analyzed by SDS-PAGE (12% gel) and Western blotting with antibodies against the indicated antigens. No binding of other proteins like synapsin could be detected. The scrambled variant binds unspecifically some SytI and Syx1. (B) Time-dependent SNARE complex formation between GST-syb2 (3 µM) and preincubated t-SNAREs (syntaxin 1, amino acids 1–262, 3 µM and SNAP25, amino acids 1–206, 15 µM) in the absence (left) and the presence (right) of the CpxII-CTD (50 µM). Complex formation was determined at 25°C for the indicated times and analyzed by SDS-PAGE. Exemplary Coomassie-stained SDS gels are shown. (C) The CpxII CTD peptide (red) slows down the time course of SNARE complex formation when compared with no peptide (SNAREs alone, black) or the scrambled peptide (green). Inset highlights delayed complex assembly in the presence of the CpxII CTD peptide (same gels as shown in B). Data were normalized to the complex formation after 240 min and averaged from the following number of trials (SNAREs, n = 8; SNAREs + CpxII CTD, n = 8; SNAREs + scr pep, n = 7). (D) Sequence alignment between CpxII CTD and the SNARE motifs of SNAP25, SybII and Syx1 (hydrophobic layer region −2 to +8, blue). The CpxII-CTD shows a high degree of similarity (underlined residues) to SNAP25-SN1 (50%, calculated for the boxed region, hydrophobic layers + 2 to+7, using the BLOSUM62 matrix). (E) Helical wheel projections of CpxII CTD (residues 117–134, boxed region in D) and SNAP25-SN1 (residues 60–77) show the amphipathic nature of the protein regions with similar hydrophobic moments (µH). Similar amino acids in the hydrophilic faces of the helices are marked with asterisks (*).