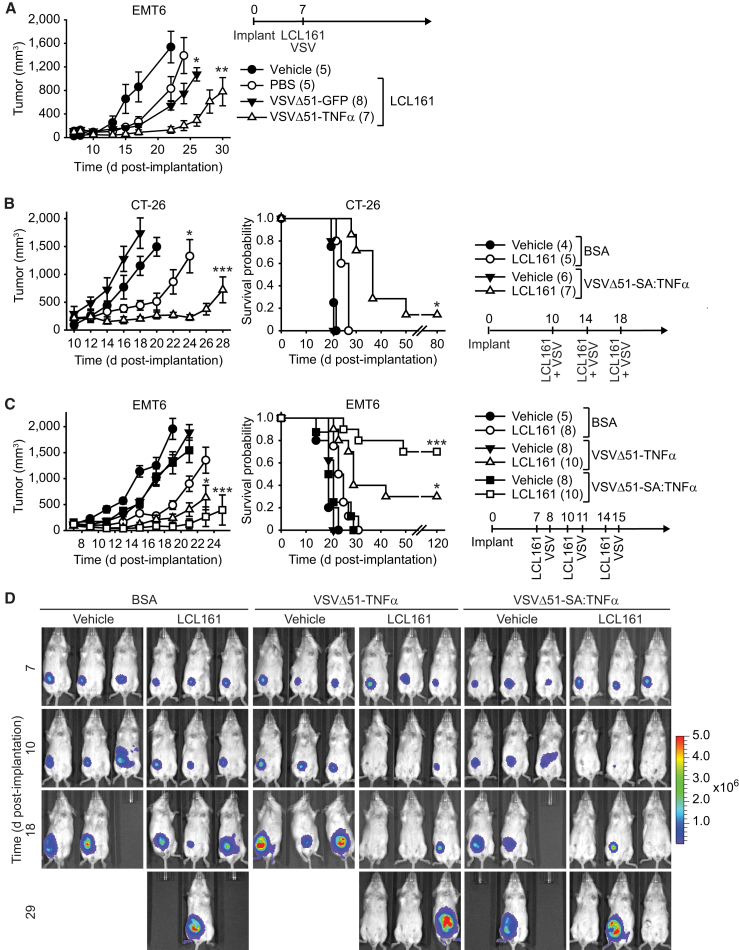
Figure 5.
TNF-α-Armed Oncolytic VSV Potently Synergize with SMC In Vivo
(A) Mice with established ∼100 mm3 mammary tumors were treated with vehicle or 50 mg/kg of the SMC LCL161 (oral gavage) and 1 × 107 PFU of VSVΔ51-GFP or VSVΔ51-TNF-α (intravenous). (B) Mice bearing ∼200 mm3 CT-26 tumors were treated with vehicle or 50 mg/kg LCL161 (oral gavage) and 1 × 107 PFU of VSVΔ51-SA:TNF-α intratumorally. (C) Mice bearing ∼100 mm3 EMT6-Fluc tumors were treated with vehicle or 50 mg/kg of LCL161 (oral gavage) and 1 × 107 PFU of VSVΔ51-TNF-α or VSVΔ51-SA:TNF-α (intratumoral). (D) IVIS images of representative EMT6-Fluc-bearing mice described in (B). Scale, p/s/cm2/sr (photons per second per square centimeter per steradian). (A–C) Left panel depicts tumor growth (mean, SEM); right panel represents the Kaplan-Meier curve depicting mouse survival. Statistical significance of tumor growth rates was assessed by one-way ANOVA with Tukey’s multiple comparison. Log rank with Holm-Sidak multiple comparison: *p < 0.05; **p < 0.01; ***p < 0.001. Number of mice per treatment group is displayed in brackets.