Figure 6:
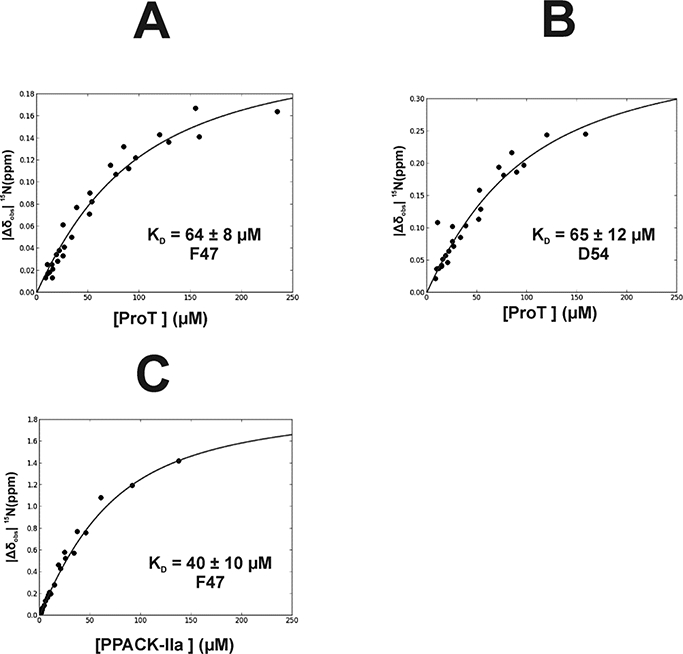
Determination of Binding Affinity (KD) for 15N-labeled F47 and D54 of PAR3GFD interacting with Prothrombin and PPACK-IIa. For this NMR titration series, the peptide ligand concentration was kept constant and the ProT and PPACK-IIa concentrations were serially diluted. As a result, the NMR titrations were measuring the binding of protein to a defined peptide concentration. (A) Interactions between ProT and PAR3G 15N-F47 led to a KD = 64 ± 8 μM, (B) ProT and PAR3G 15N-D54 led to a KD = 65 ± 12 μM, and (C) PPACK-IIa and PAR3G 15N-F47 led to a KD = 40 ± 10 μM. NMR titrations were done in triplicate. The reported KD values were determined using in-house scripts written using Python. The term |Δδobs| 15Nppm = δ15NBound - δ15Ν Free reflects the absolute difference in chemical shift between the bound and free states of the particular 15N-residue. Error analysis was carried out using a Monte-Carlo approach assuming a 10% error in the serially diluted protein samples. See Materials and Methods for more details.
