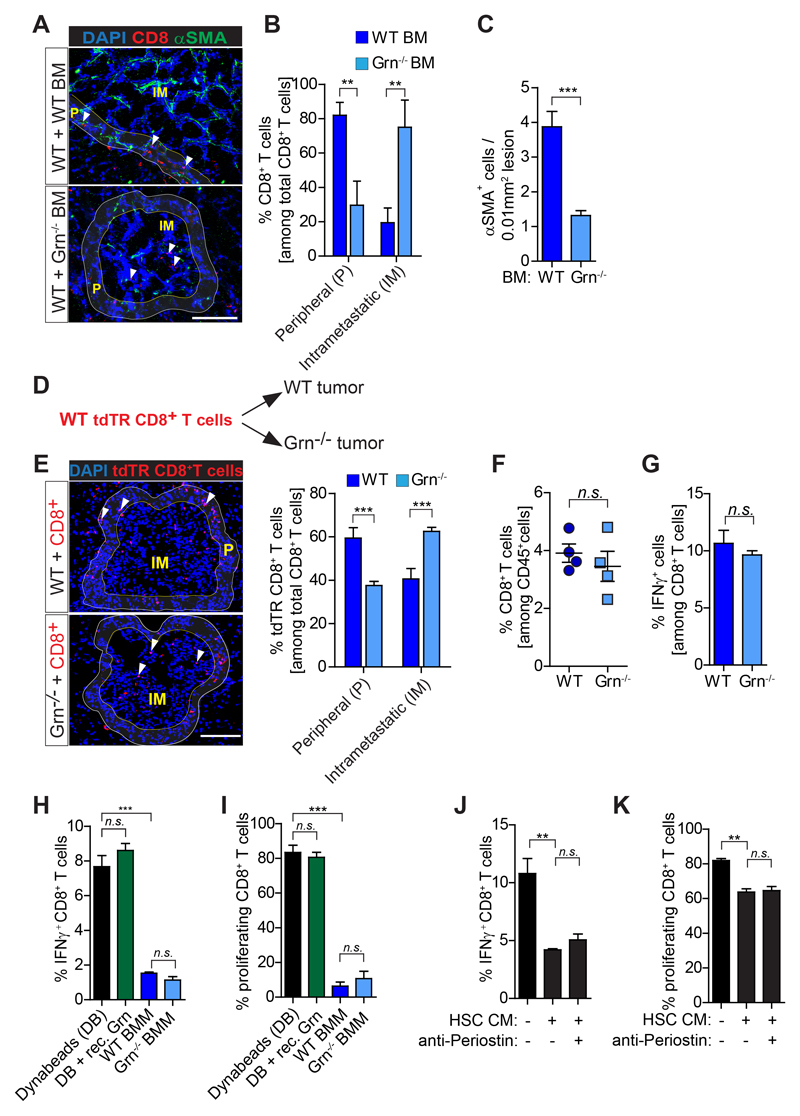
Figure 6. CD8+T cells intra-metastatic infiltration but not activity is increased in granulin depleted mice.
(A-C) Liver metastasis was induced by intrasplenically implantation of KPC cells into chimeric WT+ WT BM and WT+ Grn-/- BM mice. Entire livers were resected 14 days later and analyzed. (A) Immunofluorescence images of CD8+ T cells and αSMA+ myofibroblasts. Intrametastatic (IM) and peripheral areas (P) are indicated. (B) Quantification of peripheral (P) and intrametastatic (IM) CD8+ T cells. (C) Quantification of αSMA+ cells (WT BM, n= 5; Grn-/- BM, n= 6). (D-E) Adoptive transfer of tdTR CD8+ T cells into metastasis bearing WT and Grn-/- mice (D) Schematic illustration of the experiment. (E) Immunofluorescence images and quantification of tdTR CD8+ T cells (red) in perimetastatic (P) and intrametastatic (IM) regions (n= 3 mice / group). (F-G) KPC cells were intrasplenically implanted in mice to induce liver metastasis in WT and Grn-/- mice. Livers were resected after 14 days and analyzed (n= 4 WT and n= 4 Grn-/- mice). (F) Flow cytometry quantification of CD8+ T cell number and (G) IFNγ+ CD8+ T cell. (H-I) Bone marrow isolated macrophages (BMMs) derived from WT and Grn-/- mice and recombinant granulin (rec. Grn) were tested for the capacity of suppress splenic CD8+ T cell (H) activation (IFNγ expression levels) and (I) proliferation (CFSE dilution). (J-K) Conditioned medium (CM) generated from activated hepatic stellate cells (HSC) was used to validate the (J) activation (IFNγ expression levels) and (K) proliferation (CFSE dilution) of splenic CD8+ T cell in the presence or absence of a periostin neutralizing antibody. In H-K data are mean ± SD of 3 independent experiments. Scale bars= 100µm; ***, P<0.001; **, P < 0.01; *, P < 0.05; n.s., not significant, by unpaired t-test and/or Bonferroni multiple comparison.