Abstract
Background:
In hormone receptor-positive advanced breast cancer, a progression-free survival benefit was reported with addition of bevacizumab to first-line letrozole. However, increased toxicity was observed. We hypothesized that functional age measures could be used to identify patients at risk for toxicity while receiving letrozole plus bevacizumab for hormone receptor-positive advanced breast cancer.
Methods:
CALGB 40503 was a phase III trial that enrolled patients with hormone receptor-positive advanced breast cancer randomized to letrozole with or without bevacizumab. Patients randomized to bevacizumab were approached to complete a validated assessment tool evaluating physical function, comorbidity, cognition, psychological state, social support, and nutritional status. The relationship between pretreatment assessment measures and the incidence of grade ≥3 (National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0) adverse events was determined.
Results:
One hundred thirteen (58%) of 195 patients treated with letrozole plus bevacizumab completed the pretreatment assessment questionnaire. One patient was excluded due to missing adverse event data. The median age of patients was 56. Frequently reported grade ≥3 adverse events were hypertension (26%), pain (20%), and proteinuria (7%). Two hemorrhagic events (one grade 5) and 1 thrombosis event occurred. Age ≥65 years (p<0.01), decreased vision (p=0.04), and poorer pretreatment physical function measures (p<0.05) were found on univariate analysis to be significantly associated with increased incidence of grade ≥3 adverse events. Upon multivariate analysis, age ≥65 years (p=0.01) and decreased vision (p=0.04) remained significant. Univariable and multivariable logistic regression models demonstrated associations between age, vision, the ability to walk up flights of stairs, and grade ≥3 adverse events.
Conclusions:
Age (≥65 years), decreased vision, and impairments in physical function correlated with increased incidence of toxicity in patients receiving first-line letrozole plus bevacizumab. When evaluating therapy likely to increase toxicity, functional assessment measures can identify patients at increased risk for side effects who may benefit from closer monitoring.
Keywords: Breast Cancer, Bevacizumab, Risk Factors, Toxicity
Introduction:
Chronological age alone tells relatively little about an adult’s overall functional age. Therefore, the use of pretreatment assessments consisting of validated measures that can capture domains such as functional status, comorbid medical conditions, cognition, psychological status, social functioning and support, and nutritional status, can help to better characterize the overall functional age of an individual [1]. In addition, assessment of these domains has been reported to predict the risk of morbidity and mortality in patients with cancer undergoing systemic therapy [2–9]. This is particularly important because the findings from these assessment measures could be used to identify risk factors for treatment toxicity beyond traditional risk factors such as chronologic age.
Bevacizumab is a recombinant humanized monoclonal antibody against vascular endothelial growth factor receptor (VEGFR) that has been hypothesized to delay the emergence of resistance to endocrine therapy in patients with advanced breast cancer [10]. In the multicenter, phase III clinical trial, Cancer and Leukemia Group B (CALGB) 40503, a progression-free survival benefit was reported in patients with hormone receptor-positive advanced breast cancer treated with first-line combination bevacizumab and letrozole compared to letrozole alone [11]. However, an increase in bevacizumab-related toxicity, such as hypertension and proteinuria, was also reported with combination therapy and one (0.6%) treatment-related death due to central nervous system (CNS) hemorrhage occurred [11]. Similarly, in the phase III, multicenter letrozole/fulvestrant and avastin (LEA) trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer, eight (4.2%) treatment-related deaths were reported in the bevacizumab plus endocrine therapy treatment arm [12]. Six of the eight deaths were due to cardiovascular events and six of the eight deaths occurred in patients ≥70 years of age [12]. Based on pooled data from several prior randomized clinical trials investigating the role of bevacizumab combined with chemotherapy, a prior history of arterial thromboembolic events and older age were reported as significant risk factors for toxicity [13]. However, the identification of risk factors for toxicity to bevacizumab treatment combined with endocrine therapy in patients with advanced breast cancer has not been fully investigated.
The objective of the current study was to identify whether pre-treatment factors other than chronological age (i.e., functional status and comorbidity) may predict the risk of grade 3 or higher toxicity in patients with advanced, hormone receptor-positive breast cancer enrolled on CALGB/Alliance 40503 receiving treatment with letrozole plus bevacizumab. In addition, an exploratory analysis was performed to identify whether other factors (cognition, psychological state, social support, or nutritional status) either individually or in combination could be used to predict the risk of grade 3 or higher toxicity. Factors to be studied included cognition, pyshcological state, social support, and nutiritonal status as prior studies have demonstrated the ability of these domains to identify the risk of side effects to cancer therapy [14–31].
Patients and Methods:
Patient population
From May 2008 until November 2011, 350 patients were enrolled in the phase III multicenter CALGB 40503 clinical trial evaluating the role of letrozole with or without bevacizumab as first-line therapy for the treatment of postmenopausal women with hormone receptor-positive, locally advanced or metastatic breast cancer [11]. CALGB is now a part of the Alliance for Clinical Trials in Oncology. Eligible patients were postmenopausal (or receiving ovarian suppression with a luteinizing hormone-releasing hormone agonist) women age ≥18 years with hormone receptor-positive (defined as expressing estrogen and/or progesterone receptor ≥1% cells), locally advanced, unresectable or metastatic breast cancer. Patients were required to have Eastern Cooperative Oncology Group performance status ≤1 with adequate bone marrow, hepatic, and renal function, including urine protein dipstick grade of ≤1+ or urine protein: creatinine (UPC) ratio of <1. Key exclusion criteria for study participants included ongoing uncontrolled hypertension (blood pressure: systolic >150 mmHg and/or diastolic >90 mmHg); New York Heart Association grade ≥2 congestive heart failure; history of hypertensive crisis; history (within past 6 months) of myocardial infarction, unstable angina, stroke, abdominal fistula or abscess, or significant bleeding episode; or history of GI perforation within 12 months. The study was approved by the institutional review board at each participating institution. All participating patients completed the informed consent process.
Study schema and pretreatment patient assessment measures
Patients enrolled onto this clinical trial received treatment consisting of letrozole with or without bevacizumab. Letrozole was administered at 2.5 mg orally once per day and bevacizumab was administered at 15 mg/kg intravenously once every 3 weeks until disease progression or unacceptable toxicity. No dose reductions were permitted for letrozole or bevacizumab. Letrozole was held for grade >3 hepatic dysfunction. Bevacizumab was held for blood pressure >160/100 mmHg, urine protein ≥2 g per 24 hours or UPC ≥2, grade 3 or 4 venous thromboembolic events and for patients requiring surgery. Bevacizumab was permanently discontinued for grade ≥4 hypertension, nephrotic syndrome, reversible posterior leukoencephalopathy syndrome; grade ≥3 hemorrhage/congestive heart failure; grade ≥2 arterial thromboembolic events; any grade gastrointestinal (GI) perforation, leak, or fistula; for wound dehiscence requiring intervention; or grade ≥3 or 4 unspecified bevacizumab-related adverse events.
As part of an amendment to the clinical trial, a correlative study was added in which patients in the letrozole plus bevacizumab treatment arm of the study were asked to complete a pretreatment patient assessment questionnaire. The primary objective of this correlative study was to identify factors other than chronological age that predict the risk of grade 3, 4, or 5 toxicity in patients receiving letrozole plus bevacizumab (CONSORT diagram, Figure 1), including validated measures of functional status and comorbidity: Older Americans Resources and Services Scale (OARS)—Instrumental Activities of Daily Living [32], Medical Outcomes Study Physical Function [33], Karnofsky Performance Status Rated Health Care Professional [14], Timed “Up and Go” [15], and OARS Physical Health Section [32]. The secondary objective was to perform an exploratory analysis of whether other factors included in the patient assessments either individually or in combination predicted the risk of grade 3, 4, or 5 toxicity. These other factors included validated measures of cognition (the Blessed Orientation-Memory-Concentration [BOMC] Test [16]), psychological status [6,27], social functioning [33] and support [34], and nutritional status [30].
Fig. 1:
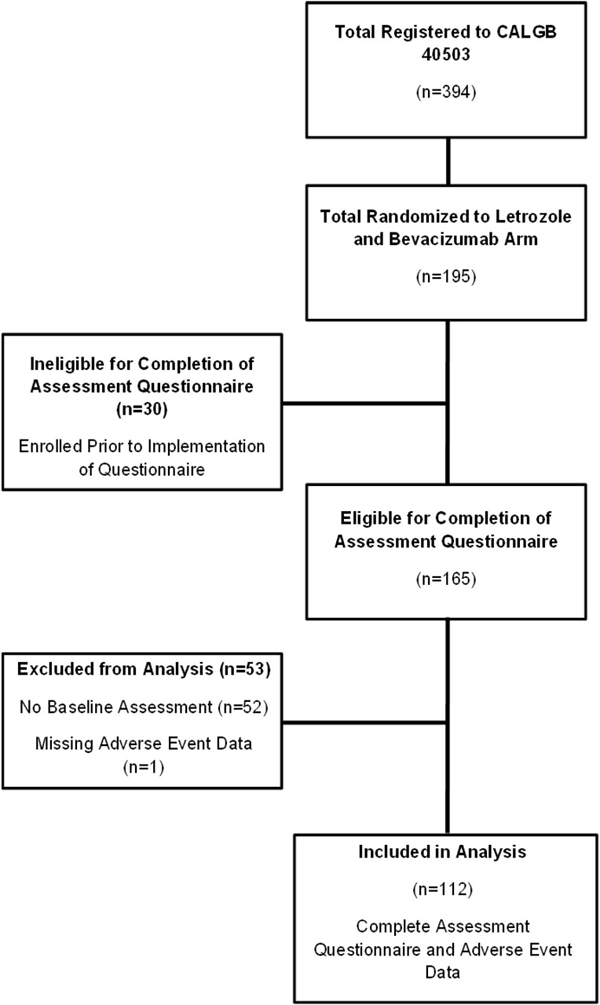
CONSORT Diagram for Letrozole and Bevacizumab Arm of CALGB 40503
Patients were followed during treatment with combination bevacizumab and letrozole. Grade ≥3 AEs as defined by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 were reported. The relationship between pretreatment patient assessment measures and the incidence of AEs was determined.
Statistical analysis
Descriptive analyses were performed to summarize patient, tumor, and treatment characteristics and pretreatment assessment results. The incidence of specific categories of NCI CTCAE grade 3, 4, or 5 toxicities were calculated. Chi square or Fisher’s exact tests [35,36], as appropriate, were used to compare baseline characteristics and incidence of AEs between patients completing the baseline assessment questionnaire versus patients with no baseline assessment questionnaire. Chi square or Fisher’s exact tests (as appropriate) [35,36], and univariable logistic regression were used to examine univariable association between the presence of grade ≥3 AEs and pretreatment assessment variables. Multivariable logistic regression was performed to determine the association of each variable in the presence of other variables. Results of the logistic models were summarized with odds radios (ORs), corresponding 95% confidence intervals (CIs), and c-statistic. All tests were two-sided, and p-values less than 0.05 were considered to be statistically significant. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Analyses were performed using SAS version 9.3 (SAS Institute INC., Cary, NC). Data were frozen on April 15, 2015. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Results:
Patient characteristics
This substudy cohort consisted of 195 patients with locally advanced or metastatic, hormone receptor-positive, breast cancer treated with first-line combination letrozole and bevacizumab. Of the 195 patients, 112 (57%) patients completed the pretreatment patient assessment questionnaire and had adverse event toxicity data available for analysis. One additional patient completed the pretreatment patient assessment questionnaire but did not have adverse event toxicity data available and was therefore excluded from these analyses. The CONSORT diagram for this study is found in Figure 1. There were no significant differences between patients completing the pretreatment assessment questionnaire compared to those who did not in terms of age, race, performance status, hormone receptor status, and incidence of grade ≥3 adverse events (data not shown). The baseline patient characteristics of the 112 patients completing the pretreatment assessment questionnaire are shown in Tables 1 and 2. The median age of participants was 56 years (range: 25–85) and 22% of patients were age 65 years or older. Sixty-three (56%) patients reported a Karnofsky performance status of 100. Seventy-six (68%) patients reported having no comorbid medical conditions and being completely independent in their instrumental activities of daily living. The median activities of daily living score (Medical Outcomes Study physical functioning) was 90 (range: 5–100). Ninety-nine (88%) patients had excellent or good vision. Only 1 patient had an abnormal BOMC Cognition Score and no patients reported greater than 5% weight loss in the past 6 months.
Table 1.
Characteristics of Patients Treated with Bevacizumab Plus Letrozole
| Characteristic | Patients Treated with Bevacizumab Plus Letrozole (n=112) |
|
|---|---|---|
| Median Age – year (range) | 56 (25–85) | |
| Age- no. (%) | <65 ≥65 |
87 (77.7%) 25 (22.3%) |
| Race- no. (%) | White Other Unknown |
103 (92.0%) 6 (5.4%) 3 (2.7%) |
| Ethnicity- no. (%) | Hispanic or Latino Not Hispanic or Latino Unknown/Not reported |
1 (0.9%) 92 (82.1%) 19 (17.0%) |
| Karnofsky Performance Status- no. (%) |
100 90 80 70 |
63 (56.3%) 35 (31.3%) 10 (8.9%) 4 (3.6%) |
| Hormone Receptor Status- no. (%) | ER+ PR+ HER2+ |
112 (100.0%) 89 (79.5%) 2 (1.8%) |
Table 2.
Assessment Variables Reported in Pretreatment Questionnaire
| Pretreatment assessment Variable | Patients Treated with Bevacizumab Plus Letrozole (n=112) |
|||
|---|---|---|---|---|
|
# of Patients (%) |
Mean ± Standard Deviation |
Median (Range) |
||
| OARS IADLa | Completely Independent | 76 (67.9%) | 13.2 ± 1.5 | 32 (6–32) |
| OARS Comorbidity | 0 1 2 or more |
76 (67.9%) 19 (17.0%) 17 (15.2%) |
||
| MOSb | Activities of Daily Living | 76.8 ± 26.5 | 90 (5–100) | |
| Social Activity | 47.9 ± 9.6 | 50 (25–75) | ||
| Timed Up and Go | Seconds | 13.0 ± 9.2 | 10 (2–60) | |
| Falls in past 6 months | None 1 or more Unavailable |
88 (78.6%) 22 (19.6%) 2 (1.8%) |
||
| Hearing |
Excellent/Good Fair/Poor/Deaf Unavailable |
97 (86.6%) 14 (12.5%) 1 (0.9%) |
||
| Vision | Excellent/Good Fair/Poor/Blind Unavailable |
99 (88.4%) 12 (10.7%) 1 (0.9%) |
||
| MHIc | Depression and Anxiety | 78.3 ± 33.0 | 81 (21–100) | |
| BOMCd Cognition Score |
<11 ≥11 Unavailable |
108 (96.4%) 1 (0.9%) 3 (2.7%) |
||
| BMI (kg/m²)e | <22 ≥30 Unavailable |
17 (15.2%) 46 (41.1%) 49 (43.7%) |
||
| Weight Loss | Greater than 5% in the last 6 months |
0 (0.0%) | ||
Factors associated with treatment toxicity to combination letrozole and bevacizumab
As previously reported with the main results of CALGB/Alliance 40503[11], treatment with bevacizumab in addition to letrozole was associated with an increase in grade ≥3 toxicities compared to treatment with letrozole alone. In the current substudy of 112 patients who completed the pretreatment assessment questionnaire and were treated with combination letrozole and bevacizumab, treatment-related grade 3 or grade 4 adverse events occurred in 55 (49%) and 5 (4%) patients, respectively. One treatment-related death also occurred as a result of CNS hemorrhage. Notable grade ≥3 adverse events occurring in this study population likely related to bevacizumab treatment include hypertension (26%), pain (20%), proteinuria (7%), syncope (3%), cardiac ischemia (1%), hemorrhage (2%), and thrombosis (1%). Additional grade ≥3 adverse events are shown in Table 3.
Table 3.
Frequent and Notable Grade ≥3 Adverse Events
| Type of Adverse Event |
Incidence (%) |
||
|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | |
| Hypertension | 27 (24%) | 2 (2%) | 0 (0%) |
| Pain | 22 (20%) | 0 (0%) | 0 (0%) |
| Proteinuria | 8 (7%) | 0 (0%) | 0 (0%) |
| Nausea | 5 (4%) | 0 (0%) | 0 (0%) |
| Syncope | 3 (3%) | 0 (0%) | 0 (0%) |
| Cardiac Ischemia | 1 (1%) | 0 (0%) | 0 (0%) |
| Hemorrhage | 1 (1%) | 0 (0%) | 1 (1%) |
| Thrombosis | 1 (1%) | 0 (0%) | 0 (0%) |
| Hypocalcemia | 0 (0%) | 1 (1%) | 0 (0%) |
| Neutropenia | 0 (0%) | 1 (1%) | 0 (0%) |
| Other Neurologic Event | 0 (0%) | 1 (1%) | 0 (0%) |
In order to identify potential risk factors for toxicity to combination treatment with letrozole and bevacizumab, univariate analyses were conducted to examine the association between patient characteristics, pretreatment assessment variables, and any grade 3–5 toxicity (See Table 4). Age (p<0.01), decreased vision (p=0.04), lower instrumental activities of daily living scores (OARS IADL) (p=0.02), and lower activities of daily living scores (MOS physical functioning) (p=0.02) were associated with grade ≥3 toxicity to treatment with letrozole and bevacizumab. In addition, specific measures such as needing help getting to places out of walking distance (p=0.02), limitation in climbing one (p=0.04) or multiple (p=0.02) flights of stairs, and limitation with walking more than one mile (p=0.04) were also associated with grade ≥3 toxicity. In multivariate analysis, factors that remained associated with grade 3 or more toxicity included age ≥65 years (p=0.01) and decreased vision (p=0.04).
Table 4.
Significant Baseline Risk Factors for Grade ≥3 Toxicity: Univariable Analysis
| Risk Factors | p-value |
|---|---|
| Age | <0.01 |
| Decreased Vision | 0.04 |
| Lower Instrumental Activities of Daily Living Scores (OARS IADLa) | 0.02 |
| Lower Activities of Daily Living Scores (MOSb) | 0.02 |
| Needing help getting to places out of walking distance* | 0.02 |
| Limitation in climbing flights of stairs** | 0.02 |
| Limitation climbing one flight of stairs** | 0.04 |
| Limitation walking more than one mile** | 0.04 |
The associations between various model variables were also performed to assess the relationship between different pretreatment assessment measures of interest with age (See Table 5). Univariable models were then developed and limitations in climbing flights of stairs (OR 3.14, c-statistic=0.635) and walking more than one mile (OR 2.67, c-statistic 0.617) were found to be more strongly associated with toxicity than age (OR 3.93, c-statistic 0.597) as a risk factor for development of grade ≥3 adverse events to treatment with letrozole and bevacizumab (See Table 6). Multivariable models with age were then performed and the addition of decreased vision and functional variables such as needing help getting to places out of walking distance, limitation in climbing flights of stairs, and limitation in walking more than one mile, all improved the model’s ability to predict grade ≥3 adverse event risk to treatment with letrozole and bevacizumab compared to age alone (See Table 7).
Table 5.
Association Between Model Variables of Interest
| Age | Vision | Needing help getting to places out of walking distancea |
Limitation in climbing several flights of stairsb |
Needing help to take medicationsa |
Limitation in walking more than one mileb |
|
|---|---|---|---|---|---|---|
| Age | - | 0.82 | <0.01 | 0.02 | 0.05 | <0.01 |
| Vision | - | 0.03 | <0.01 | 0.60 | <0.01 | |
| Needing help getting to places out of walking distancea |
- | <0.01 | <0.01 | <0.01 | ||
| Limitation in climbing several flights of stairsb |
- | 0.02 | <0.01 | |||
| Needing help to take medicationa |
- | 0.06 | ||||
| Limitation in walking more than one mileb |
- |
Table 6.
Univariable Models
| Risk Factors for Grade ≥3 Toxicity | OR (95% CI) | p-value | c-statistic |
|---|---|---|---|
| Age (≥65) | 3.93 (1.24–9.31) | 0.02 | 0.597 |
| Vision (fair or worse) | 4.70 (0.98–22.58) | 0.05 | 0.562 |
| Needing help getting to places out of walking distancea | 5.28 (1.11–25.06) | 0.04 | 0.570 |
| Limitation in climbing several flights of stairsb | 3.32 (1.41–6.99) | < 0.01 | 0.635 |
| Limitation in walking more than one mileb | 2.67 (1.21–5.87) | 0.01 | 0.617 |
Table 7.
Multivariable Models with Age
| Risk Factors for Grade ≥3 Toxicity | OR (95% CI) | p-value | c-statistic |
|---|---|---|---|
| Age (≥65) Vision (fair or worse) |
3.62 (1.30 – 10.09) 5.36 (1.10 – 26.29) |
0.01 0.04 |
0.646 |
| Age (≥65) Needing help getting to places out of walking distancea |
2.71 (0.96 – 7.69) 4.00 (0.81 – 19.74) |
0.06 0.09 |
0.632 |
| Age (≥65) Limitation in climbing flights of stairsb |
2.88 (1.02 – 8.16) 2.80 (1.24 – 6.35) |
0.05 0.01 |
0.670 |
| Age (≥65) Limitation in walking more than one mileb |
2.82 (1.00 – 7.97) 2.28 (1.01 – 5.32) |
0.05 0.05 |
0.659 |
Discussion:
The current study identified patient characteristics that may predict for grade 3 or higher toxicity in postmenopausal patients with advanced, hormone receptor-positive breast cancer receiving first-line treatment with letrozole plus bevacizumab. In addition to chronologic age alone, this study demonstrated that decreased vision and decreased physical functioning measures such as lower OARS IADL or lower MOS physical functioning scores were associated with increased grade ≥3 toxicity to treatment with letrozole and bevacizumab on univariate analysis. On multivariate analysis, only increased age and decreased vision remained associated with grade ≥3 toxicity to treatment with letrozole and bevacizumab. However, on multivariable modeling, the addition of functional variables to age was able to improve the model’s ability to predict grade ≥3 adverse event risk to treatment with letrozole and bevacizumab.
Prior research focused on identifying potential risk factors for increased toxicity to treatment with bevacizumab has been limited. In patients with various malignancies treated with chemotherapy plus bevacizumab, reported risk factors for bevacizumab treatment-related toxicity included older age, history of uncontrolled hypertension, significant cardiac disease, a history of bleeding, and a history of arterial thrombotic events [37–41]. However, chronologic age alone is often insufficient to fully describe the overall potential vulnerabilities of an individual receiving cancer therapy. The use of comprehensive assessments that include an evaluation of functional status, comorbid medical conditions, cognitive function, nutritional status, social support and psychological state can help to identify additional risk factors other than chronological age that may predict for toxicity to cancer treatments. For example, Repetto et al. demonstrated that use of comprehensive assessments can uncover problems not detected by the routine history and physical examination performed by a treating physician at time of an initial consultation or follow-up care [42]. Furthermore, the use of comprehensive assessments has also been shown to predict toxicity to chemotherapy [43,44] and survival [2].
Mohile et al. performed an analysis of the relationship between a similar pretreatment assessment questionnaire as used in the current study (consisting of domains measuring functional status, comorbid medical conditions, cognition, psychological status, social functioning and support, and nutritional status) and grade 3–5 toxicity specifically in older adults ≥65 years of age with advanced stage colorectal cancer and non-small cell lung cancer treated with chemotherapy and bevacizumab [45]. Interestingly, age was not associated with toxicity in that study and none of the additional pretreatment assessment variables were found to be specifically associated with grade 3–5 toxicity in bivariate and multivariate analysis [45]. In contrast to prior studies and the Mohile et al. study, our study focused on patients with advanced breast cancer undergoing treatment with letrozole plus bevacizumab and found that in addition to increased chronologic age, pretreatment assessment measures such as decreased vision and limitation in physical function measures were associated with increased risk of grade ≥3 toxicity. Decreased vision in this study was self-reported. Other studies based on self-reported measures found that decreased vision is likely to be associated with other comorbidities including difficulty breathing, depression, diabetes, and heart problems [46–48]. Furthermore, in a cross-sectional study, visual impairment was shown to be characterized by more medical comorbidities in comparison to non-visually impaired controls and these differences were not accounted for by age alone [49]. Therefore, it is likely that self-reported decreased vision is associated with additional medical comorbidities that increase the susceptibility for treatment-related toxicity. To our knowledge, our study is unique in that it is the first study to identify potential risk factors beyond traditional variables such as chronological age alone in predicting toxicity for patients with advanced breast cancer receiving first-line letrozole plus bevacizumab.
There were several limitations to this study. First, this was a relatively young, selective group of patients with a median age of 56, and only 25 (22%) patients were age 65 years or older. In addition, due to trial eligibility criteria, most patients had a good performance status, with over 87% of patients having a KPS ≥90. Furthermore, 68% of patients did not have any comorbid medical conditions. Therefore, the use of a pretreatment assessment questionnaire typically aimed at identifying vulnerabilities in a more diverse older adult population may not have been able to differentiate the subtle differences in patient characteristics in this relatively young, healthy, homogeneous study population. The incidence of grade ≥3 toxicity in patients treated with combination letrozole plus bevacizumab on CALGB/Alliance 40503 was also modest, consisting mainly of hypertension and proteinuria. Only 1 episode of hemorrhage, 1 episode of thrombosis, and 1 treatment-related death occurred. This is in sharp contrast to the 8 treatment-related deaths that occurred in the LEA study, which consisted of an older patient population with a median age of 64 [including 89 (47%) patients ≥65 years] treated with endocrine therapy plus bevacizumab [12]. Interestingly, 6 of the 8 patients who died in the LEA study were older adults with several comorbidities [12]. Therefore, application of a pretreatment assessment questionnaire in a more vulnerable older adult patient population such as the LEA study could potentially have been able to identify possible additional risk factors of toxicity to combination treatment with endocrine therapy and bevacizumab. Finally, in the current study many of the pretreatment assessment variables were found to be strongly associated with age, causing difficulty in building a comprehensive multivariable model. This was an exploratory analysis and larger studies in other tumor types evaluating the role of pretreatment patient assessment measures to identify risk factors for toxicity in patients undergoing treatment with combination therapy with bevacizumab will be needed in the future.
Despite these limitations, our current study further adds to the body of literature by identifying additional potential risk factors of toxicity for patients undergoing treatment with bevacizumab, which had not been previously well described. The current study demonstrated through both univariable and multivariable models that the addition of functional variables to age improved the model’s ability to predict grade ≥3 adverse event risk to treatment with letrozole and bevacizumab compared to age alone even in this relatively young, healthy, homogenous study population. This suggests that incorporation of functional age assessment measures can be used to identify potential patients at serious risk of toxicity and should potentially be considered for inclusion in future studies.
In conclusion, older age, decreased vision, and impairment in physical function correlate with increased incidence of toxicity in postmenopausal, advanced, hormone receptor-positive, breast cancer patients receiving first-line treatment with letrozole plus bevacizumab. When evaluating therapy likely to increase toxicity, functional assessment measures can be used to further identify patients at increased risk for side effects who may benefit from closer monitoring.
Acknowledgments
ClinicalTrials.gov Identifier: NCT00601900
Compliance with Ethical Standards
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), P30CA033572, U10CA180790, U10CA180795, U10CA180820, U10CA180836, U10CA180838, U10CA180857, U10CA180858, U10CA180867, U10CA180888 and CA180858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. CALGB/Alliance 40503 was supported in part by Genentech and by a grant from The Breast Cancer Research Foundation.
Footnotes
Conflict of Interest: Debu Tripathy has received remuneration from Novartis and serves in a consultant/advisory role for Novartis, Pfizer, and Nektar. Maura N. Dickler serves in a consultant/advisory role for Genentech/Roche, Pfizer, Novartis, Eli Lilly, AstraZeneca, TapImmune, and GI Therapeutics. Arti Hurria serves in a consultant/advisory role for Pierian Biosciences and MJH Healthcare Holdings, LLC and has received funding from Celgene and Novartis.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent: Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Data Availability: The analyzed datasets are available from the corresponding author upon reasonable request.
References:
- 1.Rodin MB, Mohile SG (2007) A practical approach to geriatric assessment in oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25 (14):1936–1944. doi: 10.1200/JCO.2006.10.2954 [DOI] [PubMed] [Google Scholar]
- 2.Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, Cigolari S, Rosetti F, Piazza E, Robbiati SF, Bertetto O, Novello S, Migliorino MR, Favaretto A, Spatafora M, Ferrau F, Frontini L, Bearz A, Repetto L, Gridelli C, Barletta E, Barzelloni ML, Iaffaioli RV, De Maio E, Di Maio M, De Feo G, Sigoriello G, Chiodini P, Cioffi A, Guardasole V, Angelini V, Rossi A, Bilancia D, Germano D, Lamberti A, Pontillo V, Brancaccio L, Renda F, Romano F, Esani G, Gambaro A, Vinante O, Azzarello G, Clerici M, Bollina R, Belloni P, Sannicolo M, Ciuffreda L, Parello G, Cabiddu M, Sacco C, Sibau A, Porcile G, Castiglione F, Ostellino O, Monfardini S, Stefani M, Scagliotti G, Selvaggi G, De Marinis F, Martelli O, Gasparini G, Morabito A, Gattuso D, Colucci G, Galetta D, Giotta F, Gebbia V, Borsellino N, Testa A, Malaponte E, Capuano MA, Angiolillo M, Sollitto F, Tirelli U, Spazzapan S, Adamo V, Altavilla G, Scimone A, Hopps MR, Tartamella F, Ianniello GP, Tinessa V, Failla G, Bordonaro R, Gebbia N, Valerio MR, D’Aprile M, Veltri E, Tonato M, Darwish S, Romito S, Carrozza F, Barni S, Ardizzoia A, Corradini GM, Pavia G, Belli M, Colantuoni G, Galligioni E, Caffo O, Labianca R, Quadri A, Cortesi E, D’Auria G, Fava S, Calcagno A, Luporini G, Locatelli MC, Di Costanzo F, Gasperoni S, Isa L, Candido P, Gaion F, Palazzolo G, Nettis G, Annamaria A, Rinaldi M, Lopez M, Felletti R, Di Negro GB, Rossi N, Calandriello A, Maiorino L, Mattioli R, Celano A, Schiavon S, Illiano A, Raucci CA, Caruso M, Foa P, Tonini G, Curcio C, Cazzaniga M (2005) Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23 (28):6865–6872. doi: 10.1200/JCO.2005.02.527 [DOI] [PubMed] [Google Scholar]
- 3.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. (2004) Prognostic importance of comorbidity in a hospital-based cancer registry. Jama 291 (20):2441–2447. doi: 10.1001/jama.291.20.2441 [DOI] [PubMed] [Google Scholar]
- 4.Satariano WA, Ragland DR (1994) The effect of comorbidity on 3-year survival of women with primary breast cancer. Annals of internal medicine 120 (2):104–110 [DOI] [PubMed] [Google Scholar]
- 5.Wadley VG, Okonkwo O, Crowe M, Ross-Meadows LA (2008) Mild cognitive impairment and everyday function: evidence of reduced speed in performing instrumental activities of daily living. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 16 (5):416–424. doi: 10.1097/JGP.0b013e31816b7303 [DOI] [PubMed] [Google Scholar]
- 6.Hurria A, Li D, Hansen K, Patil S, Gupta R, Nelson C, Lichtman SM, Tew WP, Hamlin P, Zuckerman E, Gardes J, Limaye S, Lachs M, Kelly E (2009) Distress in older patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27 (26):4346–4351. doi: 10.1200/JCO.2008.19.9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I (2006) Social networks, social support, and survival after breast cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24 (7):1105–1111. doi: 10.1200/JCO.2005.04.2846 [DOI] [PubMed] [Google Scholar]
- 8.Waxler-Morrison N, Hislop TG, Mears B, Kan L (1991) Effects of social relationships on survival for women with breast cancer: a prospective study. Social science & medicine 33 (2):177–183 [DOI] [PubMed] [Google Scholar]
- 9.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr., Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. The American journal of medicine 69 (4):491–497 [DOI] [PubMed] [Google Scholar]
- 10.Borgstrom P, Gold DP, Hillan KJ, Ferrara N (1999) Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer research 19 (5B):4203–4214 [PubMed] [Google Scholar]
- 11.Dickler MN, Barry WT, Cirrincione CT, Ellis MJ, Moynahan ME, Innocenti F, Hurria A, Rugo HS, Lake DE, Hahn O, Schneider BP, Tripathy D, Carey LA, Winer EP, Hudis CA (2016) Phase III Trial Evaluating Letrozole As First-Line Endocrine Therapy With or Without Bevacizumab for the Treatment of Postmenopausal Women With Hormone Receptor-Positive Advanced-Stage Breast Cancer: CALGB 40503 (Alliance). J Clin Oncol 34 (22):2602–2609. doi: 10.1200/JCO.2015.66.1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M, Loibl S, von Minckwitz G, Morales S, Martinez N, Guerrero A, Anton A, Aktas B, Schoenegg W, Munoz M, Garcia-Saenz JA, Gil M, Ramos M, Margeli M, Carrasco E, Liedtke C, Wachsmann G, Mehta K, De la Haba-Rodriguez JR (2015) Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33 (9):1045–1052. doi: 10.1200/JCO.2014.57.2388 [DOI] [PubMed] [Google Scholar]
- 13.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J, Hurwitz H (2007) Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99 (16):1232–1239 [DOI] [PubMed] [Google Scholar]
- 14.Yates JW, Chalmer B, McKegney FP (1980) Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 45 (8):2220–2224 [DOI] [PubMed] [Google Scholar]
- 15.Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society 39 (2):142–148 [DOI] [PubMed] [Google Scholar]
- 16.Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R (1995) Reliability of the Blessed Telephone Information-Memory-Concentration Test. Journal of geriatric psychiatry and neurology 8 (4):238–242. doi: 10.1177/089198879500800408 [DOI] [PubMed] [Google Scholar]
- 17.Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E (2005) Physical and mental health status of older long-term cancer survivors. Journal of the American Geriatrics Society 53 (12):2145–2152. doi: 10.1111/j.1532-5415.2005.00507.x [DOI] [PubMed] [Google Scholar]
- 18.Stafford RS, Cyr PL (1997) The impact of cancer on the physical function of the elderly and their utilization of health care. Cancer 80 (10):1973–1980 [DOI] [PubMed] [Google Scholar]
- 19.Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, De Cataldis G, Iannelli A, Bilancia D, Belli M, Massidda B, Piantedosi F, Comella G, De Lena M (2000) Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 18 (13):2529–2536. doi: 10.1200/JCO.2000.18.13.2529 [DOI] [PubMed] [Google Scholar]
- 20.Extermann M, Balducci L, Lyman GH (2000) What threshold for adjuvant therapy in older breast cancer patients? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 18 (8):1709–1717. doi: 10.1200/JCO.2000.18.8.1709 [DOI] [PubMed] [Google Scholar]
- 21.Firat S, Bousamra M, Gore E, Byhardt RW (2002) Comorbidity and KPS are independent prognostic factors in stage I non-small-cell lung cancer. International journal of radiation oncology, biology, physics 52 (4):1047–1057 [DOI] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Neville BA, Koppert LB, Lemmens VE, Tilanus HW, Coebergh JW, Weeks JC, Earle CC (2006) Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24 (26):4277–4284. doi: 10.1200/JCO.2005.05.0658 [DOI] [PubMed] [Google Scholar]
- 23.Birim O, Kappetein AP, van Klaveren RJ, Bogers AJ (2006) Prognostic factors in non-small cell lung cancer surgery. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 32 (1):12–23. doi: 10.1016/j.ejso.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 24.Zauderer M, Patil S, Hurria A (2009) Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast cancer research and treatment 117 (1):205–210. doi: 10.1007/s10549-008-0116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauvaget C, Yamada M, Fujiwara S, Sasaki H, Mimori Y (2002) Dementia as a predictor of functional disability: a four-year follow-up study. Gerontology 48 (4):226–233. doi:58355 [DOI] [PubMed] [Google Scholar]
- 26.Dodge HH, Kadowaki T, Hayakawa T, Yamakawa M, Sekikawa A, Ueshima H (2005) Cognitive impairment as a strong predictor of incident disability in specific ADL-IADL tasks among community-dwelling elders: the Azuchi Study. The Gerontologist 45 (2):222–230 [DOI] [PubMed] [Google Scholar]
- 27.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB (1998) Depressive symptoms and physical decline in community-dwelling older persons. Jama 279 (21):1720–1726 [DOI] [PubMed] [Google Scholar]
- 28.Kornblith AB, Herndon JE 2nd, Zuckerman E, Viscoli CM, Horwitz RI, Cooper MR, Harris L, Tkaczuk KH, Perry MC, Budman D, Norton LC, Holland J, Cancer, Leukemia Group B (2001) Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer 91 (2):443–454 [DOI] [PubMed] [Google Scholar]
- 29.Kornblith AB, Herndon JE, 2nd, Weiss RB, Zhang C, Zuckerman EL, Rosenberg S, Mertz M, Payne D, Jane Massie M, Holland JF, Wingate P, Norton L, Holland JC (2003) Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer 98 (4):679–689. doi: 10.1002/cncr.11531 [DOI] [PubMed] [Google Scholar]
- 30.Landi F, Zuccala G, Gambassi G, Incalzi RA, Manigrasso L, Pagano F, Carbonin P, Bernabei R (1999) Body mass index and mortality among older people living in the community. Journal of the American Geriatrics Society 47 (9):1072–1076 [DOI] [PubMed] [Google Scholar]
- 31.Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, Rodin M, Panageas KS, Holland JC, Saltz L, Kris MG, Noy A, Gomez J, Jakubowski A, Hudis C, Kornblith AB (2005) Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 104 (9):1998–2005. doi: 10.1002/cncr.21422 [DOI] [PubMed] [Google Scholar]
- 32.Fillenbaum GG, Smyer MA (1981) The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journal of gerontology 36 (4):428–434 [DOI] [PubMed] [Google Scholar]
- 33.Stewart AL WJ (1992) Measuring functioning and well-being: The Medical Outcomes Study Approach Duke University Press, Durham, NC [Google Scholar]
- 34.Sherbourne CD, Stewart AL (1991) The MOS social support survey. Social science & medicine 32 (6):705–714 [DOI] [PubMed] [Google Scholar]
- 35.Fleiss JL (1981) Statistical Methods for Rates and Proportions Second Edition. Wiley, John and Sons, Incorporated, New York, N.Y., [Google Scholar]
- 36.Altman DG (1990) Practical Statistics for Medical Research Taylor & Francis, [Google Scholar]
- 37.Selle F, Emile G, Pautier P, Asmane I, Soares DG, Khalil A, Alexandre J, Lhomme C, Ray-Coquard I, Lotz JP, Goldwasser F, Tazi Y, Heudel P, Pujade-Lauraine E, Gouy S, Tredan O, Barbaza MO, Ady-Vago N, Dubot C (2016) Safety of bevacizumab in clinical practice for recurrent ovarian cancer: A retrospective cohort study. Oncology letters 11 (3):1859–1865. doi: 10.3892/ol.2016.4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England journal of medicine 350 (23):2335–2342. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 39.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd, Eastern Cooperative Oncology Group Study E (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25 (12):1539–1544. doi: 10.1200/JCO.2006.09.6305 [DOI] [PubMed] [Google Scholar]
- 40.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine 355 (24):2542–2550. doi: 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 41.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. The New England journal of medicine 357 (26):2666–2676 [DOI] [PubMed] [Google Scholar]
- 42.Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, Parodi S, Dal Lago D, Gioia F, Monfardini S, Aapro MS, Serraino D, Zagonel V (2002) Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 20 (2):494–502. doi: 10.1200/JCO.2002.20.2.494 [DOI] [PubMed] [Google Scholar]
- 43.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP (2011) Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29 (25):3457–3465. doi: 10.1200/JCO.2011.34.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ 3rd, Balducci L (2012) Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118 (13):3377–3386. doi: 10.1002/cncr.26646 [DOI] [PubMed] [Google Scholar]
- 45.Mohile SG, Hardt M, Tew W, Owusu C, Klepin H, Gross C, Gajra A, Lichtman SM, Feng T, Togawa K, Ramani R, Katheria V, Hansen K, Hurria A, Cancer, Aging Research G (2013) Toxicity of bevacizumab in combination with chemotherapy in older patients. The oncologist 18 (4):408–414. doi: 10.1634/theoncologist.2012-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein JE, Massof RW, Deremeik JT, Braudway S, Jackson ML, Kehler KB, Primo SA, Sunness JS (2012) Baseline traits of low vision patients served by private outpatient clinical centers in the United States. Archives of ophthalmology (Chicago, Ill : 1960) 130 (8):1028–1037. doi: 10.1001/archophthalmol.2012.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitson HE, Steinhauser K, Ammarell N, Whitaker D, Cousins SW, Ansah D, Sanders LL, Cohen HJ (2011) Categorizing the effect of comorbidity: a qualitative study of individuals’ experiences in a low-vision rehabilitation program. Journal of the American Geriatrics Society 59 (10):1802–1809. doi: 10.1111/j.1532-5415.2011.03602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Nispen RM, de Boer MR, Hoeijmakers JG, Ringens PJ, van Rens GH (2009) Co-morbidity and visual acuity are risk factors for health-related quality of life decline: five-month follow-up EQ-5D data of visually impaired older patients. Health and quality of life outcomes 7:18. doi: 10.1186/1477-7525-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Court H, McLean G, Guthrie B, Mercer SW, Smith DJ (2014) Visual impairment is associated with physical and mental comorbidities in older adults: a cross-sectional study. BMC medicine 12:181. doi: 10.1186/s12916-014-0181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


