Abstract
Staphylococcus aureus (S. aureus), an important opportunistic pathogen in human and animal, causes a series of diseases in the impairing of immunity of host and even then death. Alpha-hemolysin (Hla), a primary virulence factor, plays a major role in the pathogenic progress of S. aureus, especially in pneumonia. Prim-O-glucosylcimifugin (POG), a nature chromone compound, is an active ingredient in many Chinese Medicines. In this study, POG investigated the inhibitory effect of the secretion of Hla in S. aureus strain USA300 at the subinhibitory concentrations. The hemolysis assays and western blotting assays showed that POG can decrease the production of Hla in the USA300 growth cell cultures in a dose-dependent manner. The results of RT-PCR revealed that reduction of Hla was related to inhibit the transcription of hla and RNAIII. In the cells experiment, POG was proved to protect A549 cells from Hla-medicated injury. In conclusion, POG was shown the capacity of decreased the production of S. aureus Hla. POG can be developed as a candidate agent to treat S. aureus infections against Hla.
1. Introduction
Staphylococcus aureus (S. aureus), a common Gram-positive bacterium pathogen in clinic, can cause various infectious diseases in skin, respiratory system, and bloodstream in human and animals [1]. S. aureus infection is an important zoonosis which impacts both human and animals. USA300 strain, a strain of community-associated methicillin-resistant S. aureus (MRSA), was first identified in 1998 in USA [2]. USA300 strain is causing diseases in many countries and regions around the world over the last decade [3–5]. S. aureus USA300 has become one of the most globally spread MRSA strain and caused a worldwide epidemic such as skin infection, soft tissue infections, and severe pneumonia [4]. In general, conventional antimicrobials are used to treat the bacterial diseases by killing the bacteria or inhibiting bacteria generation. Antibiotics increase selective pressure in sensitive bacteria, screen out resistant strains, and accelerate the spreading on global [6]. With widespread application and abusing of antibiotics, bacterial resistances are becoming a growing problem. With the widespread MRSA epidemics, MRSA is listed as a “serious threat” in CDC (Center for Disease Control and Prevention) [7]. Consequently, novel agents and therapeutic strategies are urgently needed to treat bacterial infections, especially of antibiotic-resistant bacteria.
It was proved that the pathogenicity of S. aureus is related to the virulence factors of S. aureus [1]. Montgomery et al. (2008) have proved that USA300 isolates had stronger pathogenicity and caused more severe pneumonia in rat pneumonia model than USA400 isolates [8]. Virulence factors, like Panton-Valentine leukocidin and alpha-hemolysin (Hla), were increasingly secreted in USA300 isolates [8, 9]. Hla is an important virulence protein that is secreted by S. aureus into the extracellular. Hla causes disease by damaging various cells and tissues [10, 11]. Pneumocytes are effective target cells of Hla [11, 12]. Many researches have proved that Hla plays an important role in the pathogenesis of S. aureus infections, especially in pneumonia [10, 13, 14]. Consequently, Hla is considered a candidate drug target for the treatment of MRSA infections such as deadly staphylococcal pneumonia [15]. Due to the indispensable character of Hla in the pathogenicity of S. aureus, drugs reacting on the Hla will be novel medicines in S. aureus infection.
As a familiar chromone, prim-O-glucosylcimifugin (POG, chemical structure shown in Figure 1) is one of Saposhnikovia divaricata (Turcz) Schischk's major effective components. In Chinese Pharmacopoeia, POG has been defined as index component and active ingredient standardized of Saposhnikovia divaricata (Turcz) Schischk [16, 17]. Previous researches showed that POG exhibited many potent pharmacological activities including anticancer, analgesic, anticonvulsant, antipyretic, antinociceptive, and anti-inflammatory effects [18–21]. Saposhnikovia divaricata (Turcz) Schischk was commonly used for treatment of common colds and headache in traditional Chinese medicine. To our knowledge, no study had shown the effects of POG on the expression of Hla in S. aureus. In this study, we evaluated the effect of POG on the inhibition of Hla secretion in S. aureus USA300 using the hemolysis assay, western blotting, RT-PCR, and cell experiments.
Figure 1.
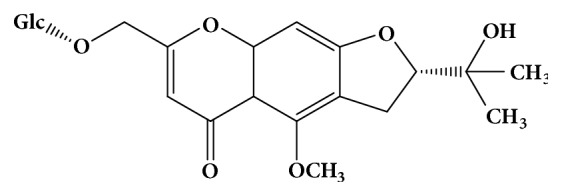
The chemical structure of prim-O-glucosylcimifugin (CAS No: 80681-45-4).
2. Materials and Methods
2.1. Bacterial Strains, Cell Line, and Reagents
The community-associated methicillin-resistant S. aureus (CA-MRSA) strain USA300 (ATCC BAA-1717), a Hla-producing strain, was used in this study. This strain was purchased from the American Type Culture Collection (ATCC). In this cells experiment, A549 cells (human alveolar epithelial cell line, ATCC CCL185) were used and commercially obtained from ATCC. Prim-O-Glucosylcimifugin (POG, purity≥98%) was obtained from Chengdu Herbpurify Co., Ltd., (Chengdu, China). The solution (40960 μg/mL) of POG was prepared by dimethyl sulfoxide (DMSO; Sigma-Aldrich) and stored in 4°C for in vitro study.
2.2. Susceptibility Testing
The minimal inhibitory concentrations (MICs) of POG against S. aureus strain were determined by the broth microdilution method which recommended by the Clinical and Laboratory Standards Institute. Shortly, POG was diluted to the concentration range of 2-512 μg/mL in a 96-well plate by double dilution method. Bacteria (5 × 105 CFU/mL) were inoculated to each well. The plate was inoculated at 37°C for 24 h. The MIC was defined as the lowest drug concentration that inhibited bacteria growth and was repeated for three times.
2.3. Growth Curve Assay
Bacteria were cultured in TSB at 37°C to OD600 = 0.3 and equally (100 ml) divided into 7 flasks (250 mL), followed by the accretion of POG at concentrations of 0, 4, 8, 16, 32, 64, and 128 μg/mL. These flasks were placed in an incubator with 37°C and constant shaking (200 rpm) under aerobic conditions. Bacteria growth was determined by reading the OD600 values of the cultures every 30 min.
2.4. Hemolysis Assay
Bacteria were grown in TSB at 37°C, with different concentrations of POG. When bacteria reached the postexponential growth phase (OD600 of 2.5), cell culture was centrifuged (1000×g, room temperature, 2 min). The supernatants were collected and stored in sterile tube. Twenty-five μL defibrinated rabbit red cells and 100 μL of supernatant were added to 875 μL sterile PBS. The mixtures were tenderly mixed well and incubated at 37°C chamber for 15 min, and then the mixtures were centrifuged by 10,000×g at 20°C for 1 min. The OD543 values of the supernatant of mixtures were measured by spectrophotometer (Agilent Technologies). The hemolytic activities were reflected by the OD543 values. Negative control (without POG) was served as 100% hemolysis. The hemolysis percentage of drug group was calculated by comparison with drug-free control.
2.5. Western Blotting
The bacteria culture supernatants, which were collected from the hemolysis assay, were used for western blotting analysis. The protocol of the western blotting was performed as described previously [22]. The proteins in the supernatants were separately denatured via boiling with Laemmli SDS buffer for 5 min. Subsequently, target protein was separated by sodium dodecyl sulfate (SDS) and polyacrylamide (12%) gel. Hla was detected using ECL western blotting detection reagents (Bio-Rad, ChemiDoc™ MP). The antibody against S. aureus Hla and the secondary antibodies (an anti-rabbit antiserum conjugated with horseradish peroxidase) were obtained from Sigma-Aldrich.
2.6. RNA Isolation and RT-PCR Assay
Bacteria cultures were the same of the hemolytic activity assay. Bacteria were centrifuged by 5000×g at 4°C for 5 min. The cell pellets were resuspended and lysed in TES buffer. Total RNAs of samples were extracted by using the RNeasy mini kit (Qiagen) according to the manufacturer's instructions. DNA was removed from each RNA preparation using RNase-free DNase I (Qiagen) according to the manufacturers' directions for rigorous DNase treatment. The OD260 of the purified RNA was measured by a UV spectrophotometer (Agilent Technologies). cDNA was synthetized from extracted RNA using iScript cDNA synthesis Kit (Bio-Rad). PCR amplification was assessed by Real-Time System (CFX ConnectTM, Bio-Rad Laboratories, Inc., USA). All samples were analyzed in triplicate. The 16S rRNA was used (housekeeping gene) as a reference gene. In this study, the relative expression of a target gene versus the 16S rRNA gene was utilized to determine the changes in the transcript-level between samples. The results were analyzed with ABI Prism 7000 SDS software. PCRs were performed with the primers that are listed in Table 1.
Table 1.
Primers used in real-time RT-PCT.
| Primer | Sequence | Location within gene |
|---|---|---|
| 16S rRNA-forward | 5′-GCTGCCCTTTGTATTGTC-3′ | 287-305 |
| 16S rRNA-reverse | 5′-AGATGTTGGGTTAAGTCCC-3′ | 446-465 |
| hla-forward | 5′-TTGGTGCAAATGTTTC-3′ | 485-501 |
| hla-reverse | 5′-TCACTTTCCAGCCTACT-3′ | 569-586 |
| RNAIII-forward | 5′-TTCACTGTGTCGATAATCCA-3′ | 367-386 |
| RNAIII-reverse | 5′-GGAAGGAGTGATTTCAATGG-3′ | 428-447 |
2.7. Viability and Cytotoxicity Assay
A549 cells (ATCC CCL 185) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) contained heat-inactivated fetal calf serum (10%, Bioind) and penicillin/streptomycin (100 U/ml, Sigma). Cells were seeded on 96-well culture plates at a cells concentration of 2.0 × 105 cells each well for 18 h at 37°C incubator with 5% CO2. S. aureus USA300 was multiplied in TSB at 37°C. When the OD600 of bacterial cultures were 0.5, bacteria were collected from 5 ml culture medium by centrifuge (1 min, 1000×g, 4°C). The pellets were washed with sterile PBS three times. And then, the bacteria were resuspended in 10 ml of DMEM (without penicillin/streptomycin) to get bacterial suspension. One hundred μL bacterial suspensions were added to each well seeded with A549 cells in 96-cell dishes. A549 cells with bacteria were cultured 6 h at 37°C with indicated concentrations of POG. The morphology and growth condition of A549 were determined via the live/dead (green/red) reagent (Invitrogen) and by Cytotoxicity Detection kit (LDH) (Roche, Switzerland), respectively. The microscopic images of stained cells were obtained using a confocal laser scanning microscope (Nikon, Japan). The amounts of released LDH in mix-culture were measured by a microplate reader (Tecan, Austria) at an absorbance of 490 nm. All experiments were accorded manufacturers' directions.
2.8. Statistical Analysis
The results were analyzed for significance using an independent Student's t-test, and a P value of < 0.05 was considered to be statistically significant. The statistical analyses were performed using the SPSS 13.0 statistical software.
3. Results
3.1. Effect of Prim-O-Glucosylcimifugin on S. aureus Growth
The minimal inhibitory concentration (MIC) of POG against S. aureus USA300 was 128 μg/mL. Additionally, we detected the growth curve of S. aureus USA300 treated with different concentrations of POG (4 to 32 μg/mL). The growth curves showed that POG did not inhibit the multiplication of bacterium in POG-treated groups and negative group (Figure 2). These data showed that POG had a little anti-S. aureus activity, but there were no influence on the multiplication of S. aureus in the range of experimental concentration.
Figure 2.
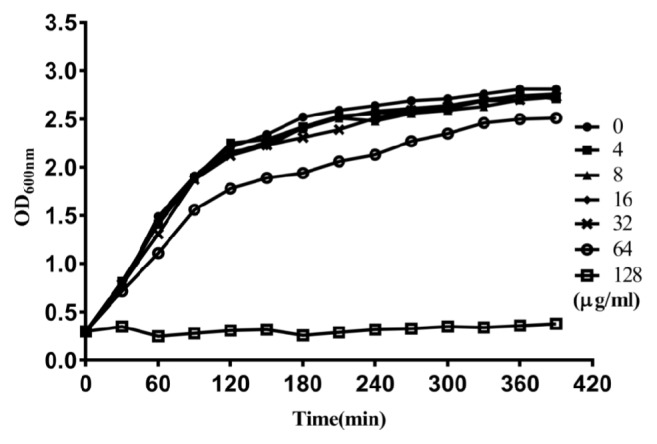
Growth curves of S. aureus USA300 strain in TSB with or without POG. Symbols ●, ■, ▲, ◆, x, ○, and □ represent S. aureus USA300 strain grown in TSB with 0, 4, 8, 16, 32, 64, and 128 μg/mL of POG, respectively.
3.2. Prim-O-Glucosylcimifugin Inhibits the Hemolytic Activity of Coculture Supernatant
Hemolytic activities of bacteria supernatants cocultured with or without POG were investigated using the hemolysis of rabbit blood red cells. Figure 3 showed that POG inhibited the hemolytic activity in a dose-response relationship (from 4 to 32 μg/mL) in coculture supernatants. When USA300 was cultured with 16 μg/mL of POG, the hemolysis value of culture supernatant was 29.3% (P < 0.01) compared with the negative control. Almost no hemolysis (3.2%) was detected at the concentration of 32 μg/mL. Additionally, POG did not lyse rabbit blood red cells and had no effect on the hemolysis induced by pure Hla (Sigma-Aldrich) with the concentration up to 64 μg/mL (date not shown). The hemolytic activity was inhibited by POG in USA300 culture supernatant with a dose-response manner in this study.
Figure 3.
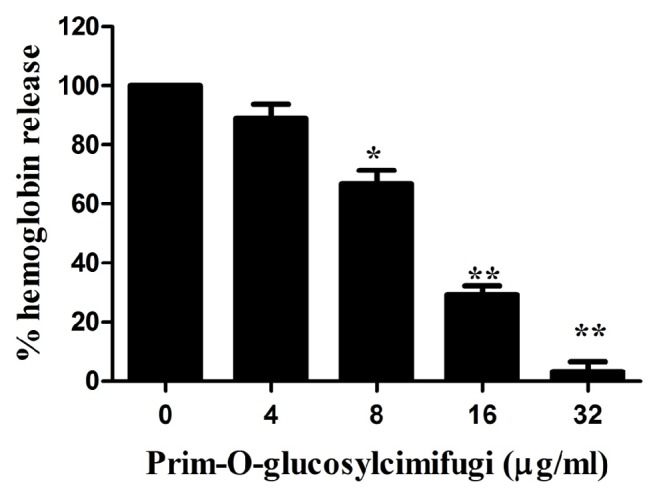
Hemolytic activity of Hla produced by S. aureus USA300 coculture with subinhibitory concentrations of prim-O-glucosylcimifugin. The data shown are representative of three independent experiments. ∗ indicates P < 0.05 and ∗∗ indicates P < 0.01, compared with the prim-O-glucosylcimifugin-free culture.
3.3. Prim-O-Glucosylcimifugin Decreases the Secretion of Hla in Culture Supernatant
The relation between decreased hemolysis and the content of Hla in culture medium was confirmed via western blotting assay. In accordance with the hemolysis assay, POG decreased the secretion of Hla in a concentration-dependent manner (Figure 4). A recognizable reduction in Hla secretion was observed in the group that treated with 16 μg/mL of POG. Nevertheless, almost no target protein was observed in S. aureus USA300 treated with 32 μg/mL POG.
Figure 4.

The production of α-hemolysin (Hla) in the culture supernatants of S. aureus USA300 grown with or without prim-O-glucosylcimifugin.
3.4. Prim-O-Glucosylcimifugin Attenuates hla and RNAIII Transcription in S. aureus
Alpha-hemolysin is coded by the hla gene. The real-time RT-PCR was used to detect the transcriptions of hla in S. aureus USA300 treated with POG doses ranging from 4 to 32 μg/mL. The arg regulatory system is a positive regulator of Hla in S. aureus. In S. aureus, the products of more than 100 virulence factors (including Hla) were positively associated with RNAIII, an effector molecule of the agr system [23, 24]. The transcription of RNAIII was also investigated. Our results showed that the transcriptions of hla and RNAIII were reduced in S. aureus USA300 treated with POG by a dose-dependent relationship (Figure 5). When USA300 was cultured with POG in the concentration of 32 μg/mL, the relative transcription levels of hla and RNAIII in USA300 were observably decreased (5.5% and 9.8% for hla and RNAIII, respectively) compared with negative control group.
Figure 5.
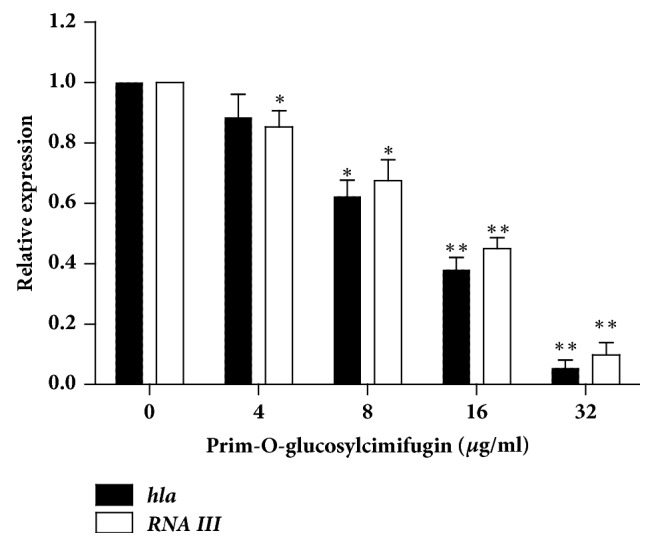
The relative expression of hla and RNAIII in S. aureus USA300 after growing in the absence or in the presence prim-O-glucosylcimifugin. The data shown are representative of three independent experiments. ∗ indicates P < 0.05 and ∗∗ indicates P < 0.01, compared with the prim-O-glucosylcimifugin-free culture.
3.5. Prim-O-Glucosylcimifugin Alleviates Hla-Mediated A549 Cells Injury
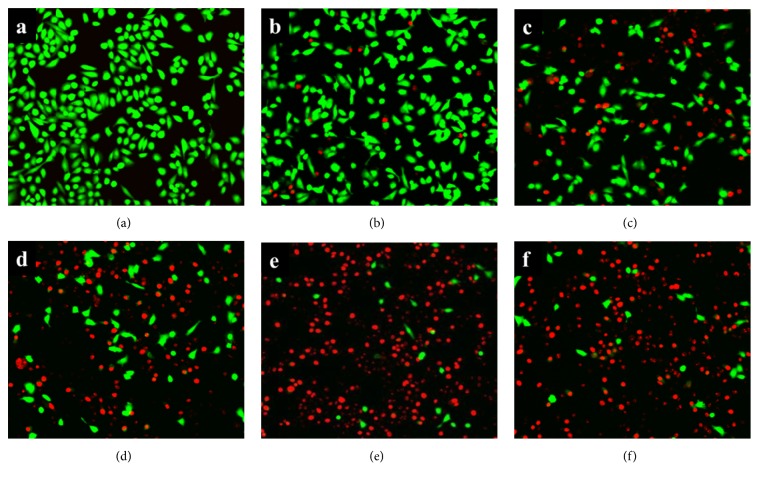
Human alveolar epithelial A549 cell is usually used as cell model for pulmonary diseases in laboratory experiment [25]. Previous researches have indicated that Hla secreted by S. aureus is the main caused of cell injury in S. aureus and A549 cells coculture system [26, 27]. The potential protection of POG on Hla-mediated cell injury was verified in the S. aureus USA300 and A549 cells coculture system with different concentrations of POG. After being stained with the live/dead reagent, the live and dead cells showed green and red fluorescence, respectively. A549 cells without S. aureus showed green fluorescence in confocal laser scanning microscope (Figure 6(a)). In the drug-free coculture system, the rate of cell death significantly increased compared with A540 cells (without S. aureus). Under the confocal laser scanning microscope, more red fluorescent cells appeared in the drug-free coculture system (Figure 6(f)), also in the lowest drug concentration (Figure 6(e)). However, the amount of red fluorescence was significantly decreased in the coculture system treated with 8 μg/mL of POG (Figure 6(d)) and treated with 16 μg/mL of POG (Figure 6(c)). POG showed protection for A549 cells in 32 μg/mL, as shown by almost no notice of red fluorescence (Figure 6(b)).
Figure 6.
The situation of live and dead cells in the A549 human alveolar epithelial cell coculture with S. aureus USA300 treated with different concentrations of prim-O-glucosylcimifugin. Live/dead reagent-stained A549 was observed with fluorescent imaging (100×), using calcein AM and ethidium homodimer-1 (EthD-1), respectively. (a) Uninfected A549 cells; (b) A549 cells was infected by S. aureus USA300 with 32 μg/ml of POG; (c) A549 cells was infected by S. aureus USA300 with 16 μg/ml of POG; (d) A549 cells was infected by S. aureus USA300 with 8 μg/ml of POG; (e) A549 cells was infected by S. aureus USA300 with 4 μg/ml of POG; (f) A549 cells was infected by S. aureus USA300 without POG.
When the cell walls are destroyed by Hla, lactate dehydrogenase (LDH) will release to the culture medium. There is proportionality between the content of LDH in culture medium and the degree of cells damage. The concentration of LDH in coculture mediums was quantitatively estimated by LDH release assay kit. The amounts of LDH in the mediums showed the effect of POG to protect the influence of S. aureus USA300 on A549 cells. The results are presented as percentages of cell death. A dose-dependent reduction was shown at the concentrations ranging from 4 to 32 μg/ml of POG in the coculture systems (Figure 7). The level of LDH release was 94.8% in the coculture system without POG. However, the level of cell death was decreased to 5.3% in the system treated with 32 μg/mL of POG. Previous experiments demonstrated that POG is not impacting the growth of S. aureus. Consequently, it can be concluded that POG protected A549 cells by inhibiting the Hla production and decreasing S. aureus CFUs.
Figure 7.
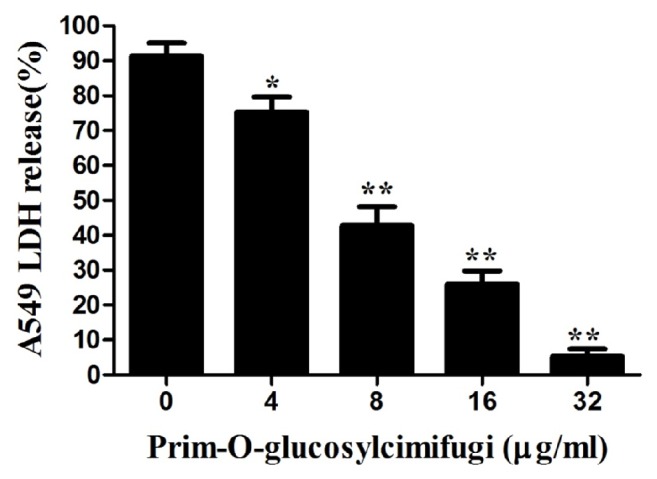
LDH release by A549 cells was quantified using a microplate reader at 490 nm. The samples were tested after exposure to certain concentrations of prim-O-glucosylcimifugin. The data are displayed as the means from three independent experiments. ∗P < 0.05 and ∗∗P < 0.01, compared with prim-O-glucosylcimifugin-free culture.
4. Discussion
The discovery of antibiotics and other antimicrobial therapies is one of major medical advances in modern medicine. Antibiotics have saved a great many people, particularly in bacterial infections. However, the extensive and long-term antibiotics use was leading to a remarkable increase in antibiotic resistance among bacteria, as antibiotics are increasing the survival selective pressure on the growth of bacteria. In clinic, more antibiotics or higher doses are used to treat drug-resistant bacterial infections. This situation were more costly in treatment or more toxic for patients [28, 29]. With the increasing resistance and the lack of development of new antibiotics, drug-resistant bacterial infections have become a major global health trouble. New drugs, especially drugs for new targets and new mechanisms are in urgent need of resistance bacterial infections. In this study, we found that POG can inhibit S. aureus reproduction under the high concentration (MIC=128 μg/mL) and reduced the production of Hla at the low concentration. POG could be an ideal lead compound for anti-S. aureus infections.
MRSA is a familiar type of bacteria that is resistant to several antibiotics. The MRSA was identified as one of six different to treat ESKAPE pathogens (Enterococcus, Staphylococcus, Klebsiella, Acinetobacter, Pseudomonas, and Enterobacter) by the Antimicrobial Availability Task Force of the Infectious Diseases Society of America [30]. In 1961, after the application of methicillin (1960), MRSA isolates were first reported by British scientists [31]. Soon afterwards, MRSA was widespread worldwide [32, 33]. The CDC reported that two percent of people carry MRSA. In clinic, MRSA infections are treated with antibiotics. With a range of antibiotics application, MRSA is resistant to many common practice antibiotics. That means MRSA infections can be more difficult to treat than nonresistance bacteria. According to WHO, it is considered that the mortality probability of people infected with MRSA strains is higher than that of people infected with nonresistant S. aureus strains [34]. The data from CDC showed that more than 94,000 people were infected with MRSA, and the number of MRSA-related deaths is at least 19,000 people each year in USA [7]. MRSA remains an important pathogen that cause of hospital-acquired and community-associated infections worldwide. In the hospital and community, people were infected with MRSA, which led to severe diseases, such as pneumonia, bacteremia, and surgical site infections. USA300, a common community-associated MRSA strain, causes severe infection in healthy population worldwide [35–38]. CDC reported that 64% of MRSA isolates were USA300 strain in infected patients in 2006. USA300 strain, a really big issue in public health, can cause life-threatening illness.
The pathogenicity of S. aureus infection depends on the virulence factors that contain surface proteins, extracellular toxins, and enzymes produced from S. aureus. S. aureus excreted these virulence factors to assist bacteria adhesion and destruction to host cells, avoidance of the host immune defense, growth, and spread in host. Alpha-hemolysin, which is an important virulence protein, secreted by S. aureus into the extracellular, causes diseases by damaging various cells and tissues. Pneumocytes are effective target cells of Hla. Hla plays an important role in the pathogenesis of S. aureus pneumonia. Bubrck Wardenburg et al. (2007) have demonstrated that mice infected with S. aureus Hla mutation strains that were lacking in the Hla gene showed significantly less lung injury than mice infected with wild-type strains [14]. Hla is proved to be an ideal target for the drug development of therapeutics in many bacteria that secrete hemolysin [39–41]. Many studies had showed that some small molecules can control the staphylococcal aureus pneumonia by inhibiting the expression and activation of Hla in animal disease models [39, 42]. Hla is proved a virulence factor in S. aureus USA300 [43]. In our research, we found that the production of Hla was influenced by the concentration of prim-O-glucosylcimifugin. Hla productions are regulated by the accessory gene regulator (agr) quorum-sensing system in S. aureus. RNAIII, which is the major transcript of the agr operon, directly affects the expression of Hla [44]. The expression of RNAIII was also investigated in this study.
Many studies have proved that the production of Hla was increased, when S. aureus was in low concentrations of antibiotics (β-lactams and fluoroquinolones) [45, 46]. S. aureus can strengthen Gram-negative bacterial pathogenicity by its Hla in mice pneumonia model [47]. Our results have proved that POG reduced the expression of Hla. POG may have the potential for treatment mixed-infection (G- + S. aureus) combination with common antibiotics, especially in S. aureus excreted Hla.
5. Conclusion
In this study, prim-O-glucosylcimifugin reduced Hla secretion without affecting the growth of S. aureus and showed the dose-dependent manner. In the cell experiments, A549 cells were protected from Hla injured by POG. POG could be developed as an antivirulence drug used in diseases caused by Hla.
Acknowledgments
The study was funded by China Postdoctoral Science foundation (Grant no. 2015M582563) and General Project of Sichuan Provincial Department of Education (Grant no. 16ZB0036). The authors also thank Wang Jianfeng and Zhang Bing (Jilin University, College of Veterinary Medicine) for their technical assistance.
Data Availability
All relevant data are included within the paper. For further details, please contact yinlizi@hotmail.com.
Ethical Approval
The defibrinated rabbit blood was purchased from Zheng Zhou Jiu Long Biological Products Co. Ltd. and no animals were directly used in the experiments above.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Lowy F. D. Staphylococcus aureus infections. The New England Journal of Medicine. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.McDougal L. K., Steward C. D., Killgore G. E., Chaitram J. M., McAllister S. K., Tenover F. C. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the united states: Establishing a national database. Journal of Clinical Microbiology. 2003;41(11):5113–5120. doi: 10.1128/jcm.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle-Vavra S., Daum R. S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of panton-valentine leukocidin. Laboratory Investigation. 2007;87(1):3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 4.Nimmo G. R. USA300 abroad: Global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clinical Microbiology and Infection. 2012;18(8):725–734. doi: 10.1111/j.1469-0691.2012.03822.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Mee-Marquet N. L. Whole-genome sequencing analysis, an essential tool for shedding light on the obscure evolution of Staphylococcus aureus USA300. The Journal of Infectious Diseases. 2016;213(9):1362–1363. doi: 10.1093/infdis/jiv490. [DOI] [PubMed] [Google Scholar]
- 6.Rossolini G. M., Arena F., Pecile P., Pollini S. Update on the antibiotic resistance crisis. Current Opinion in Pharmacology. 2014;18:56–60. doi: 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7. CDC. http://www.cdc.gov/mrsa/community/index.html.
- 8.Montgomery C. P., Boyle-Vavra S., Adem P. V., et al. Comparison of virulence in community-associated methicillin-resistant staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. The Journal of Infectious Diseases. 2008;198(4):561–570. doi: 10.1086/590157. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery C. P., Boyle-Vavra S., Daum R. S. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0015177.e15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berube B. J., Wardenburg J. B. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins. 2013;5(6):1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong C., Neoh H.-M., Nathan S. Targeting Staphylococcus aureus toxins: A potential form of anti-virulence therapy. Toxins. 2016;8(72) doi: 10.3390/toxins8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maretzky T., Reiss K., Ludwig A., et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proceedings of the National Acadamy of Sciences of the United States of America. 2005;102(26):9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardenburg J. B., Patel R. J., Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infection and Immunity. 2007;75(2):1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardenburg J. B., Bae T., Otto M., DeLeo F. R., Schneewind O. Poring over pores: Alpha-hemolysin and panton-valentine leukocidin in Staphylococcus aureus pneumonia. Nature Medicine. 2007;13(12):1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 15.Ragle B. E., Karginov V. A., Wardenburg J. B. Prevention and treatment of Staphylococcus aureus pneumonia with a β-cyclodextrin derivative. Antimicrobial Agents and Chemotherapy. 2010;54(1):298–304. doi: 10.1128/AAC.00973-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai J., Chen X., Cheng W., et al. Sensitive liquid chromatography-mass spectrometry method for simultaneous determination of two active chromones from Saposhnikovia root in rat plasma and urine. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2008;868(1-2):13–19. doi: 10.1016/j.jchromb.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 17.He Q. J., Gao Y. H., Wei Z. X. Determination of primo-glucosylcimifugin in biyan tablets by HPLC. Zhongyao Xinyao Yu Linchuang Yaoli. 2009;3:25–28. [Google Scholar]
- 18.Deng C., Yang X., Zhang X. Rapid determination of panaxynol in a traditional chinese medicine of Saposhnikovia divaricata by pressurized hot water extraction followed by liquid-phase microextraction and gas chromatography-mass spectrometry. Talanta. 2005;68(1):6–11. doi: 10.1016/j.talanta.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Wang C.-C., Chen L.-G., Yang L.-L. Inducible nitric oxide synthase inhibitor of the Chinese herb I. Saposhnikovia divaricata (Turcz.) Schischk. Cancer Letters. 1999;145(1-2):151–157. doi: 10.1016/s0304-3835(99)00248-7. [DOI] [PubMed] [Google Scholar]
- 20.Chen N., Wu Q., Chi G., et al. Prime-O-glucosylcimifugin attenuates lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology. 2013;16(2):139–147. doi: 10.1016/j.intimp.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L., Li Y., Li Y., et al. Antinociceptive Effects of Prim-O-Glucosylcimifugin in Inflammatory Nociception via Reducing Spinal COX-2. Biomolecules & Therapeutics. 2016;24(4):418–425. doi: 10.4062/biomolther.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang P., Chen J., Sun M., et al. Imperatorin inhibits the expression of alpha-hemolysin in Staphylococcus aureus strain BAA-1717 (USA300) Antonie van Leeuwenhoek. 2016;109(7):915–922. doi: 10.1007/s10482-016-0690-9. [DOI] [PubMed] [Google Scholar]
- 23.Novick R. P., Ross H. F., Projan S. J., Kornblum J., Kreiswirth B., Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO Journal. 1993;12(10):3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenig R. L., Ray J. L., Maleki S. J., Smeltzer M. S., Hurlburt B. K. Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. Journal of Bacteriology. 2004;186(22):7549–7555. doi: 10.1128/JB.186.22.7549-7555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirst R. A., Yesilkaya H., Clitheroe E., et al. Sensitivities of human monocytes and epithelial cells to pneumolysin are different. Infection and Immunity. 2002;70(2):1017–1022. doi: 10.1128/IAI.70.2.1017-1022.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bubeck Wardenburg J., Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. The Journal of Experimental Medicine. 2008;205(2):287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang X., Yan M., Ji Y. The H35A mutated alpha-toxin interferes with cytotoxicity of staphylococcal alpha-toxin. Infection and Immunity. 2009;77(3):977–983. doi: 10.1128/IAI.00920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farbman L., Avni T., Rubinovitch B., Leibovici L., Paul M. Cost-benefit of infection control interventions targeting methicillin-resistant Staphylococcus aureus in hospitals: Systematic review. Clinical Microbiology and Infection. 2013;19(12):E582–E593. doi: 10.1111/1469-0691.12280. [DOI] [PubMed] [Google Scholar]
- 29.Antonanzas F., Lozano C., Torres C. Economic features of antibiotic resistance: The case of methicillin-resistant Staphylococcus aureus. Pharmaco Economics. 2015;33(4):285–325. doi: 10.1007/s40273-014-0242-y. [DOI] [PubMed] [Google Scholar]
- 30.Rice L. B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. The Journal of Infectious Diseases. 2008;197(8):1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 31.Rolinson G. N. “Celbenin” - resistant staphylococci. British Medical Journal. 1961;1(5219):125–126. doi: 10.1136/bmj.1.5219.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mediavilla J. R., Chen L., Mathema B., Kreiswirth B. N. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Current Opinion in Microbiology. 2012;15(5):588–595. doi: 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Chen C.-J., Huang Y.-C. New epidemiology of Staphylococcus aureus infection in asia. Clinical Microbiology and Infection. 2014;20(7):605–623. doi: 10.1111/1469-0691.12705. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Antimicrobial resistance: 2014 global report on surveillance. 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/ [Google Scholar]
- 35.Hopman J., Peraza G., Espinosa F., et al. USA300 methicillin-resistant Staphylococcus aureus in Cuba. Antimicrobial Resistance and Infection Control. 2012;1(2) doi: 10.1186/2047-2994-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fossum Moen A. E., Tannæs T. M., Leegaard T. M. USA300 methicillin-resistant Staphylococcus aureus in Norway. APMIS. 2013;121(11):1091–1096. doi: 10.1111/apm.12077. [DOI] [PubMed] [Google Scholar]
- 37.Seidl K., Leimer N., Palheiros Marques M., et al. USA300 methicillin-resistant Staphylococcus aureus in Zurich, Switzerland between 2001 and 2013. International Journal of Medical Microbiology. 2014;304(8):1118–1122. doi: 10.1016/j.ijmm.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Rao Q., Shang W., Hu X., Rao X. Staphylococcus aureus ST121: A globally disseminated hypervirulent clone. Journal of Medical Microbiology. 2015;64(12):1462–1473. doi: 10.1099/jmm.0.000185. [DOI] [PubMed] [Google Scholar]
- 39.Qiu J., Niu X., Dong J., et al. Baicalin protects mice from Staphylococcus aureus pneumonia via inhibition of the cytolytic activity of alpha-hemolysin. The Journal of Infectious Diseases. 2012;206(2):292–301. doi: 10.1093/infdis/jis336. [DOI] [PubMed] [Google Scholar]
- 40.Wang J., Qiu J., Tan W., et al. Fisetin inhibits Listeria monocytogenes virulence by interfering with the oligomerization of listeriolysin O. The Journal of Infectious Diseases. 2015;211(9):1376–1387. doi: 10.1093/infdis/jiu520. [DOI] [PubMed] [Google Scholar]
- 41.Li H., Zhao X., Wang J., et al. β-sitosterol interacts with pneumolysin to prevent Streptococcus pneumoniae infection. Scientific Reports. 2016;5(1) doi: 10.1038/srep17668.17668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soromou L. W., Zhang Y., Cui Y., et al. Subinhibitory concentrations of pinocembrin exert anti-Staphylococcus aureus activity by reducing α-toxin expression. Journal of Applied Microbiology. 2013;115(1):41–49. doi: 10.1111/jam.12221. [DOI] [PubMed] [Google Scholar]
- 43.King J. M., Kulhankova K., Stach C. S., Vu B. G., Salgado-Pabón W., Leggett J. E. Phenotypes and virulence among Staphylococcus aureus USA100, USA200, USA300, USA400, and USA600 clonal lineages. mSphere. 2016;1(3) doi: 10.1128/mSphere.00071-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng H.-L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. Journal of Bacteriology. 1988;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohlsen K., Ziebuhr W., Koller K.-P., Hell W., Wichelhaus T. A., Hacker J. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant staphylococcus aureus isolates. Antimicrobial Agents and Chemotherapy. 1998;42(11):2817–2823. doi: 10.1128/aac.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worlitzsch D., Kaygin H., Steinhuber A., Dalhoff A., Botzenhart K., Döring G. Effects of amoxicillin, gentamicin, and moxifloxacin on the hemolytic activity of Staphylococcus aureus in vitro and in vivo. Antimicrobial Agents and Chemotherapy. 2001;45(1):196–202. doi: 10.1128/AAC.45.1.196-202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen T. S., Hilliard J. J., Jones-Nelson O., et al. Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Science Translational Medicine. 2016;8(329) doi: 10.1126/scitranslmed.aad9922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included within the paper. For further details, please contact yinlizi@hotmail.com.