Fig. 8.
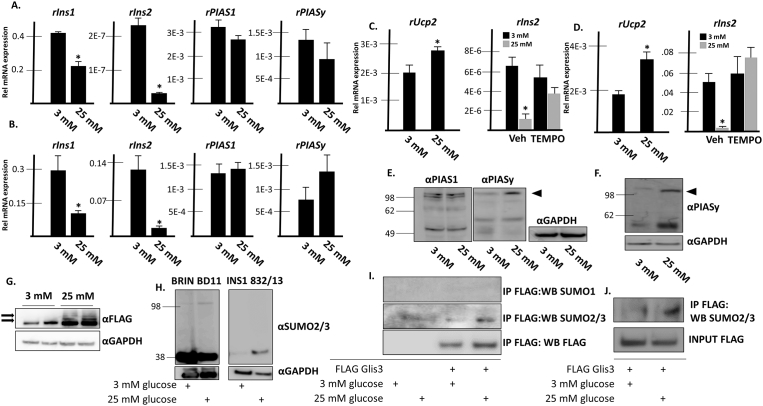
Glis3 is SUMOylated in BRIN BD11 cells under conditions of chronically elevated glucose. A. BRIN BD11 cells were grown in media containing 3 mM or 25 mM D-glucose. Media was changed after 24 h and after 48 h, total RNA was collected and the specified mRNA was measured by qRT-PCR analysis. A representative experiment is shown. Each bar represents relative mRNA levels normalized to 18s rRNA ± S.D. * indicates statistically different value compared to cells grown in 3 mM glucose p < 0.05. B. INS1 832/13 cells were grown and assayed as described in A. C. BRIN BD11 or D. INS1 832/13 cells were grown as described in A. Vehicle or 50 uM 4-hydroxy-TEMPO was additionally added to media as indicated. After 48 h, RNA was collected and qRT-PCR was performed as described in A. E. BRIN BD11 or F. INS1 832/13 cells were grown as described in A. Cells were harvested, proteins were separated by SDS-PAGE, and analyzed by Western blotting using the specified antibodies. GAPDH is shown as an internal control. G. BRIN BD11 cells stably expressing FLAG-Glis3 were grown in the presence of either 3 mM or 25 mM glucose for 48 h. Cells were harvested and proteins were separated by SDS-PAGE and subsequently analyzed by Western blotting using the indicated antibodies. Arrows indicate higher molecular weight bands that may represent SUMOylated Glis3. GAPDH is shown as an internal control. H. BRIN BD11 or INS1 832/13 cells were grown as described in A. Cells were harvested, proteins were separated by SDS-PAGE, and analyzed by Western blotting using the indicated antibodies. GAPDH is shown as an internal control. I. BRIN BD11 cells stably expressing FLAG empty vector or FLAG Glis3 were grown in media containing 3 mM or 25 mM D-glucose. After 48 h, co-immunoprecipitation was performed using an anti-M2 FLAG antibody. Immunoprecipitated and input fractions were analyzed by Western blotting using a rabbit anti-SUMO1 antibody, mouse anti-SUMO2/3 antibody, or mouse anti-M2 FLAG antibody and the appropriate HRP-conjugated secondary antibodies. J. INS1 832/13 cells were transfected with FLAG-Glis3 and grown in media containing 3 mM or 25 mM D-glucose. After 48 h, co-immunoprecipitation was performed using an anti-M2 FLAG antibody. Immunoprecipitated and input fractions were analyzed by Western blotting using a mouse anti-SUMO2/3 antibody. Unaltered blots are shown in Supplemental Fig S8.