Highlights
-
•
Myoepithelial carcinoma of the vulva and epithelioid sarcoma has overlapping histological, immunohistochemical and genetic features.
-
•
Similarities between two diagnoses in aggressive vulvar cancer should be noted as a sarcoma-based chemotherapy regimen should be considered.
-
•
We recommend immediate surgical resection with bilateral superficial and deep inguinal lymph node dissections in these aggressive cancers.
-
•
Identification of SMARCB1-deficiency trials of targeted therapies such as EZH2 inhibitors which show promise in halting further progression.
Abbreviations: CT, computed tomography; IV, intravenous; PET, Positron Emission Tomography; Gy, gray; PRC2, polycomb repressive complex 2
Keywords: Myoepithelial carcinoma, Epithelioid sarcoma, SMARCB1 deficiency, EZH2 inhibitor, Case report
Abstract
Introduction
Myoepithelial carcinoma and proximal-type epithelioid sarcoma of the vulva are two rare malignancies with known aggressive behavior. In addition to a similar clinical course, these two disease entities also have significant histologic and pathologic overlap. Given the rarity of these malignancies, there is limited literature on the appropriate treatment regimen. Nevertheless, there is a consensus that early surgical resection is beneficial in both cases.
Presentation
We present a case of a patient who was initially diagnosed with myoepithelial carcinoma of the vulva with a differential later expanded to include possible epithelioid sarcoma.
Discussion
We demonstrate the importance in early identification of a SMARCB1 deficiency. Additionally, we suggest an appropriate treatment regimen for these patients going forward. Specifically, we encourage consideration of bilateral superficial and deep inguinal lymphadenectomies. Furthermore, sarcoma based chemotherapy regimens in the appropriate clinical setting may be beneficial in treating SMARCB1 deficient tumors. Additionally, there are now clinical trials involving EZH2 Inhibitors which may offer benefit for similar patients going forward.
Conclusion
This case highlights the difficulty in making a definitive diagnosis, and the importance in identifying a SMARCB1 deficiency as it will affect treatment options and may allow for enrollment in ongoing clinical trials.
1. Introduction
Myoepithelial carcinoma and epithelioid sarcoma of the vulva are two rare cancers with overlapping features [1]. They are both characterized by aggressive growth and can respond to chemotherapy [1]. Soft tissue myoepithelial carcinoma has a heterogeneous morphology and is composed of cytologically malignant epithelioid cells arranged in cords, clusters, or sheets enmeshed in a variably myxoid or hyalinized stroma [2]. It is commonly described in the literature as a salivary tumor, with rare cases arising from the vulva [3]. In contrast, epithelioid sarcoma is a mesenchymal tumor consisting of large, polygonal, eosinophilic cells similar to carcinomas with peripheral spindling and reactivity for epithelial and mesenchymal markers [1]. It is classified into conventional and proximal variants with the proximal-type reported to arise in the vulva and behave more aggressively [1]. SMARCB1-deficiency has been identified in both cancers, making them difficult to distinguish on a genetic basis [4]. Both tumors should be widely resected with consideration of neoadjuvant or adjuvant chemotherapy [3,5].
We present a case of an aggressive vulvar cancer with an unclear diagnosis of either myoepithelial carcinoma or proximal-type epithelioid sarcoma. Our patient was initially diagnosed and treated in the community before presenting to our academic center. In this review we highlight diagnostic challenges in distinguishing between these malignancies, and discuss the potential treatment strategies. Please note, this case has been reported in line with SCARE criteria [6].
2. Case report
A 33-year-old female with no medical history presented to her gynecologist with pelvic pain. A CT scan showed a 3.6 × 3.1 cm heterogeneous right inguinal mass with a differential of inflammatory versus neoplastic lymph node. She subsequently underwent an excision and biopsy at an outside hospital.
Pathology was suggestive of myoepithelial carcinoma with cytologically malignant intermediate-sized polyhedral cells with eosinophilic cytoplasm. The stroma ranged from myxoid to hyalinized. Immunohistochemistry was positive for EMA and SMA with a minority of cells expressing keratin cocktail. Tumor cells lost expression of INI-1 and were negative for S100, CD34, SOX10, p63 and GFAP. FISH was negative for rearrangement of EWSR-1 - up to 50% of myoepithelial carcinomas lack this rearrangement [7].
She subsequently presented to our outpatient oncology clinic with swelling and severe pain at the operative site. CT imaging revealed interval growth of a dense, lobulated mass involving the right labia, extending into the subcutaneous tissues anterior to the right pubic symphysis and involving the right rectus musculature; one enlarged right inguinal lymph node was identified at 1.5 cm (Fig. 1, Fig. 2).
Fig. 1.
CT scan of abdomen and pelvis demonstrating coronal view of 14 cm right vulvar tumor extending into groin 1 week prior to surgery.
Fig. 2.
CT scan abdomen and pelvis showing axial view of 5 × 9 cm right vulvar mass 1 week prior to surgery.
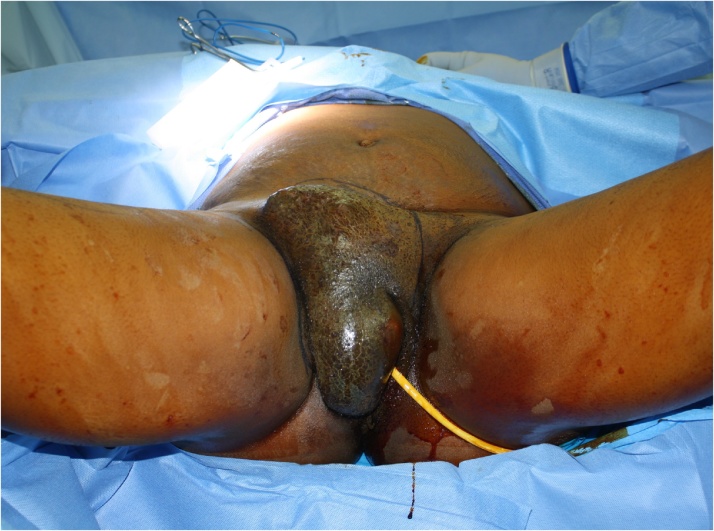
After a multidisciplinary discussion, the decision was made to proceed with neoadjuvant chemotherapy as surgical resection at this point was unlikely to result in negative margins. She received one cycle of carboplatin AUC 6 and paclitaxel 175 mg/m2. Unfortunately, the patient progressed rapidly with imminent fungation of the tumor through the skin and intractable pain. Now two months after initial surgery, she underwent resection of a 26-cm right groin mass (Fig. 3) along with a right superficial inguinal lymphadenectomy. All frozen sections of the margins were negative. The gynecology team performed a radical vulvectomy and the plastic surgery team performed reconstruction of the right groin with a pedicled right anterolateral thigh flap and right sartorius flap. The abdominal wall was reconstructed with a Strattice (LifeCell, NJ, USA) underlay mesh (Fig. 4). Histopathologic examination demonstrated high-grade myoepithelial carcinoma with necrosis and hemorrhage, venous invasion, negative surgical margins with the closest margin 0.1 cm and two lymph nodes containing small nests of metastasis. Postoperative recovery was uneventful and she was discharged post-operative day eight.
Fig. 3.
Intraoperative view of right vulvar tumor prior to resection. Tumor measurements in pathology were 26 × 7.5 × 10.5 cm (length × width × height).
Fig. 4.
Postoperative photograph after resection with radical vulvectomy, right superficial lymphadenectomy, and reconstruction using pedicled right anterolateral thigh flap and right sartorius flap.
Six weeks after surgery, chemoradiation was initiated with a plan for cisplatin 40 mg/m2 weekly for five weeks and intensity-modulated radiation therapy with sixty Gy of radiation fractionated into thirty doses, with five doses per week. She had received three doses of cisplatin chemotherapy and eleven fractions of radiation, when she developed recurrent pain, and imaging revealed recurrence in bilateral groin nodes, pelvic nodes and para-aortic nodes. Given the lack of response to carcinoma chemotherapy regimens, it was decided to treat the patient with sarcoma chemotherapy. The patient stopped radiation therapy and received ifosfamide 10 g/m2 without doxorubicin, due to risk for radiation recall. In the interim, whole genome sequencing from FoundationOne® revealed loss of SMARCB1, a gene that encodes the SNF5 protein, also known as INI-1; this was consistent with the loss of INI-1 on immunohistochemistry. Due to the lack of response to carcinoma chemotherapy regimen and deletion of SMARCB1, the possibility was raised that the patient could have proximal-type vulvar epithelioid sarcoma.
She initially responded well to ifosfamide-based chemotherapy with decreased pelvic pain. After two cycles of chemotherapy, again she had recurrence of pain along with abdominal distension and ascites likely secondary to peritoneal metastasis. The patient wished to proceed with a clinical trial of combination pazopanib plus an aurora kinase inhibitor, both targets implicated in epithelioid sarcoma. Unfortunately, she was excluded from the study due to poor performance. Per her family’s request, she was transferred back to our institution where she expired nine months after the onset of her symptoms.
3. Discussion
Epithelioid sarcomas and myoepithelial carcinomas can be difficult to distinguish due to a number of overlapping features [4]. Myoepithelial carcinomas of the vulva are extremely rare neoplasms, with aggressive behavior and a tendency to recur and metastasize [3,5]. One literature review found that most women affected by this tumor are under age 50 [3]. Similarly, proximal-type epithelioid sarcoma of the vulva is a rare soft tissue malignancy that most commonly affect the labia majora of reproductive aged women [8]. Epithelioid sarcomas also have a high recurrence rate and often metastasize early to lymph nodes [8]. One literature review of ten patients with vulvar epithelioid sarcoma who underwent radical surgery showed the median overall survival was 17.7 months, demonstrating the aggressive behavior of these tumors [8]. As both myoepithelial carcinoma and epithelioid sarcoma metastasize early, a prompt treatment plan should be initiated.
The general consensus for either type of tumor is initial management with immediate surgical resection [3,5]. The remaining questions are whether to perform a lymph node dissection and the extent of dissection. Authors of one literature review of vulvar epithelioid carcinoma suggest that lymph node dissection should be considered in the context of high clinical suspicion, but do not specify extent of dissection [8]. In our patient with one prominent lymph node of the right groin on CT scan, we performed a right superficial inguinal lymphadenectomy. However, as she later had recurrence in bilateral groin nodes it may be beneficial to perform a bilateral superficial and deep inguinal lymph node dissection in the case of a similar aggressive form of vulvar cancer.
Given the rarity of these tumors, there is no established protocol regarding neoadjuvant or adjuvant chemotherapy [3,5]. Treatment of epithelioid sarcoma can be attempted with chemotherapy agents such as ifosfamide or doxorubicin [9]. Vulvar myoepithelial carcinomas can be treated with carcinoma chemotherapeutic drugs like carboplatin and paclitaxel, although there have been reports of sensitivities to sarcoma based chemotherapy regimens as well [10,11]. Our patient’s tumor did not demonstrate a response to carboplatin, cisplatin or paclitaxel, but did show a temporary improvement from ifosfamide. As these tumors can be difficult to distinguish pathologically and both may have response to sarcoma based chemotherapy it may be useful to start with these agents, or in the minimum to keep a low threshold in switching to a sarcoma-based chemotherapy regimen.
In addition to chemotherapy, targeted therapy based on genetic markers must also be considered. Loss of SMARCB1 and INI-1 can be a characteristic of both epithelioid sarcomas and myoepithelial carcinomas of the vulva [4]. Targeted therapy to restore the function of SMARCB1/INI-1 may be helpful in treating these tumors [[12], [13], [14], [15]]. There is evidence that tumors with loss of SMARCB1 may be sensitive to targeted therapies such as inhibitors of EZH2 [[12], [13], [14], [15]]. EZH2 is the catalytic subunit of the PRC2, a histone-lysine N-methyltransferase enzyme functioning in transcriptional repression [13]. One study showed that specifically in SMARCB1-deficient tumors, inhibitors of EZH2 resulted in antiproliferative effects on tumor growth [13]. Suppression of EZH2 induced a similar function to SMARCB1, and restored tumor suppressor gene activity [13]. There is currently an ongoing EZH2 inhibitor clinical trial whose results are eagerly awaited [12]. Inhibitors of EZH2 may be the future treatment for SMARCB1-deficient tumors.
In conclusion, both myoepithelial carcinomas and epithelioid sarcomas of the vulva are very challenging to diagnose given their rarity and overlapping pathologic features. While most vulvar tumors tend to be treated with platin-based regimens, recognition of the overlap in the disease spectrum may justify attempts at sarcoma-based chemotherapy in the appropriate setting. Furthermore, while it is generally established that local excision is the appropriate first step for these tumors, one should also consider bilateral superficial and deep inguinal lymphadenectomies. It is also important to identify SMARCB1-deficiency in this subset of tumors as these patients may be able to benefit from clinical trials earlier in the treatment course, rather than waiting for progression on chemotherapy. We believe that further research is warranted regarding the efficacy of targeted therapies in these tumors going forward.
Written informed consent was unable to be obtained from the deceased patient or next-of-kin for publication of this case report and accompanying images.
Conflicts of interest
No conflicts of interest.
Sources of funding
No funding for research.
Ethical approval
Ethical approval has been given by Ethics Committee at University of Miami Hospital. Please see “Cover Letter” attachment as this includes the email from Dr. Kenneth W. Goodman, our chair of the Ethics Committee.
Consent
Our patient is deceased and despite multiple attempts we were unable to contact next of kin.
The head of our medical team/hospital or legal team have taken responsibility that exhaustive attempts have been made to contact the family and that the paper has been sufficiently anonymised not to cause harm to the patient or their family.
Author contributions
Kristina Khazeni – study concept or design, writing the paper.
Hannah Labove – writing the paper.
Dr. Danny Yakoub – study concept or design, writing the paper.
Dr. Elizabeth Paulus – data collection.
Dr. Harvey Chim – review of paper.
Dr. Breelyn Wilky – writing the paper.
Dr. Andrew Rosenberg – writing the paper.
Dr. Matthew Pearson – review of paper.
Registration of research studies
Not a research study.
Guarantor
Danny Yakoub.
Contributor Information
Kristina Khazeni, Email: kristina.khazeni@jhsmiami.org.
Hannah LaBove, Email: hannah.labove@jhsmiami.org.
Breelyn Wilky, Email: B.Wilky@med.miami.edu.
Andrew E. Rosenberg, Email: ARosenberg@med.miami.edu.
Elizabeth Paulus, Email: elizabeth.paulus@cmich.edu.
Harvey Chim, Email: HWC8@med.miami.edu.
Joseph M. Pearson, Email: MPearson@med.miami.edu.
Danny Yakoub, Email: DYakoub@med.miami.edu.
References
- 1.Armah H.B., Parwani A.V. Epithelioid sarcoma. Arch. Pathol. Lab. Med. 2009;133(5):814–819. doi: 10.5858/133.5.814. [DOI] [PubMed] [Google Scholar]
- 2.Gleason B.C., Fletcher C.D. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am. J. Surg. Pathol. 2007;31(12):1813–1824. doi: 10.1097/PAS.0b013e31805f6775. [DOI] [PubMed] [Google Scholar]
- 3.Miyata M., Hasegawa K., Ishikawa K., Kato R., Udagawa Y., Kuroda M. Primary myoepithelial carcinoma of the vulva and review of the literature. J. Obstet. Gynaecol. Res. 2011;37(6):617–622. doi: 10.1111/j.1447-0756.2010.01392.x. [DOI] [PubMed] [Google Scholar]
- 4.Folpe A.L., Schoolmeester J.K., McCluggage W.G. SMARCB1-deficient vulvar neoplasms: a clinicopathologic, immunohistochemical, and molecular genetic study of 14 cases. Am. J. Surg. Pathol. 2015;39(6):836–849. doi: 10.1097/PAS.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 5.Han C.H., Li X., Khanna N. Epithelioid sarcoma of the vulva and its clinical implication: a case report and review of the literature. Gynecol. Oncol. Rep. 2016;15:31–33. doi: 10.1016/j.gore.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Fowler A.J., Saeta A. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Rekhi B., Sable M., Jambhekar N.A. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461(6):687–697. doi: 10.1007/s00428-012-1335-7. [DOI] [PubMed] [Google Scholar]
- 8.Patrizi L., Corrado G., Saltari M. Vulvar "proximal-type" epithelioid sarcoma: report of a case and review of the literature. Diagn. Pathol. 2013;8:122. doi: 10.1186/1746-1596-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judson I., Verweij J., Gelderblom H. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 10.Noronha V., Cooper D.L., Higgins S.A., Murren J.R., Kluger H.M. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7(3):270–271. doi: 10.1016/S1470-2045(06)70619-2. [DOI] [PubMed] [Google Scholar]
- 11.Xu T., Liao Z., Tang J. Myoepithelial carcinoma of the head and neck: a report of 23 cases and literature review. Cancer Treat. Commun. 2014;2(2–3):24–29. [Google Scholar]
- 12.Ho P. A Phase II, Multicenter Study of the EZH2 Inhibitor Tazemetostat in Adult Subjects With INI1-Negative Tumors or Relapsed/Refractory Synovial Sarcoma. 2015 https://clinicaltrials.gov/ct2/show/NCT02601950 . [Google Scholar]
- 13.Knutson S.K., Warholic N.M., Wigle T.J. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc. Natl. Acad. Sci. U. S. A. 2013;110(19):7922–7927. doi: 10.1073/pnas.1303800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S.C.V., Ramachandra N. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011;71(9) doi: 10.1158/0008-5472.CAN-10-2167. [DOI] [PubMed] [Google Scholar]
- 15.Niu H., Manfredi M., Ecsedy J.A. Scientific rationale supporting the clinical development strategy for the investigational Aurora A kinase inhibitor alisertib in cancer. Front. Oncol. 2015;5:189. doi: 10.3389/fonc.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]