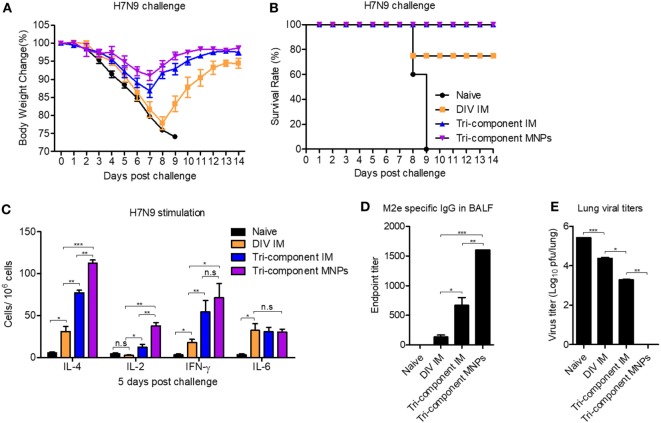
Figure 5.
Protection efficacy against a heterologous influenza virus challenge. BALB/c mice (N = 5) were intramuscular (IM) immunized with divalent inactivated vaccine (DIV), tri-component vaccine, or microneedle patch (MNP) immunized with the tri-component vaccine. Four weeks after the immunization, mice were challenged with 2 × LD50 of A/Shanghai/02/2013 (H7N9, rSH) reassortant virus. Body weight changes (A) and survival rates (B) were monitored daily for 14 days. Mice were sacrificed at day 5 post challenge for the collection of spleens, bronchoalveolar lavage fluids (BALF), and lungs. (C) Cytokine-secreting cells in splenocytes were measured in the presence of 4 µg/ml purified A/Shanghai/02/2013 (H7N9, rSH) virus by ELISPOT. (D) The levels of M2e-specific IgG in BALF. (E) Lung viral titers. Data represent mean ± SEM. The statistical significance was analyzed by Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, n.sp > 0.05.