Figure 7.
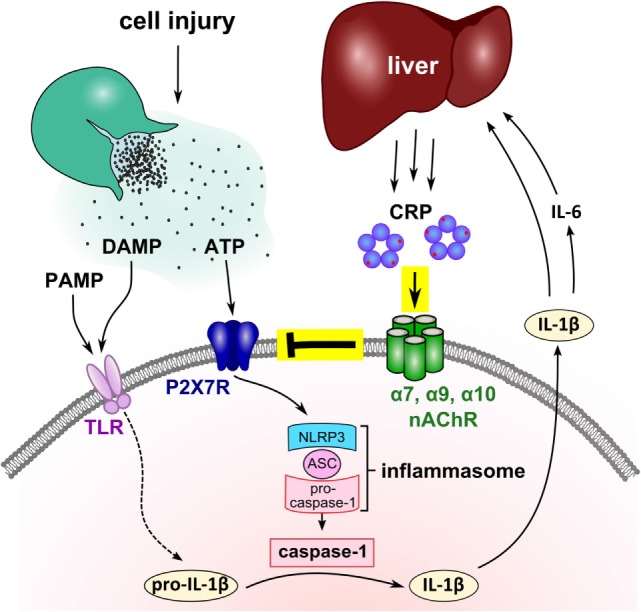
Suggested mechanism of the mutual control of C-reactive protein (CRP) and interleukin-1β (IL-1β) during trauma-associated sterile inflammation. Injury causes the release of cytoplasmic danger-associated molecular patterns (DAMP) and ATP, and frequently microbiota, a source of pathogen-associated molecular patterns (PAMP), get access to the damaged tissue. DAMP and PAMP can bind to pattern recognition receptors such as toll-like receptors (TLR) at monocytic cells and induce the synthesis of pro-IL-1β, the inactive cytoplasmic precursor of IL-1β. Extracellular ATP activates the P2X7R and leads to inflammasome assembly and caspase-1 activation. Activated caspase-1 cleaves pro-IL-1β and enables the release of mature bioactive IL-1β that in turn, induces IL-6. IL-1β and IL-6 activate the hepatic synthesis of CRP and blood levels of CRP quickly rise to 1,000-fold. We demonstrated that purified human endogenous CRP inhibits the response of P2X7R to ATP via nAChR subunits α7, α9, and α10. The function of CRP critically depends on its association with small molecules such as phosphocholine (red dots) that seem to mediate the interaction with nicotinic receptors. We suggest that the CRP-mediated control of ATP-induced IL-1β release is a negative feedback loop that controls excessive systemic inflammation in response to injury. Although the nAChR is illustrated as a pentamer, its molecular structure remains to be elucidated.
