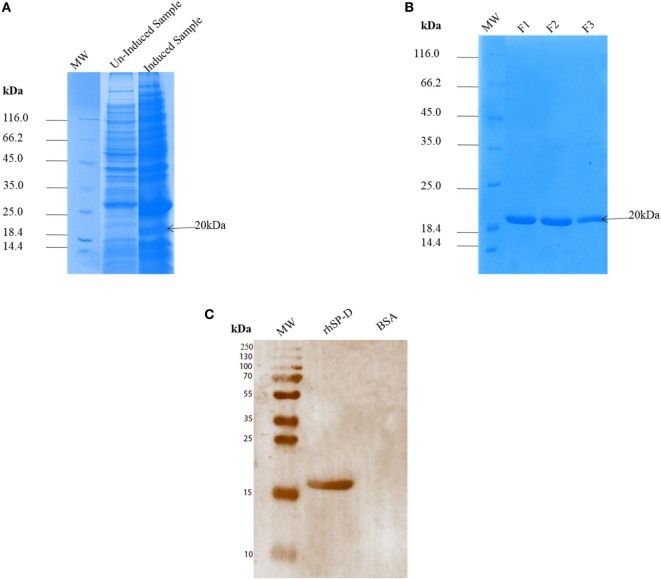
Figure 1.
SDS-PAGE (12% v/v) under reducing conditions showing expression and purification of a recombinant surfactant protein D (rfhSP-D). The neck and carbohydrate recognition domain regions were expressed in Escherichia coli BL21 (λDE3) pLysS. (A) Following induction with 0.5 mM IPTG, a ~20 kDa band appeared being overexpressed compared to uninduced sample. Following denaturation–renaturation cycle, the rfhSP-D was purified on an affinity column to homogeneity after elution with EDTA as fractions F1, F2 and F3 (B). A rabbit polyclonal antibody raised against full-length SP-D purified from human bronchoalveolar lavage (C) recognized the purified rfhSP-D, but not BSA that was used as a negative control protein.