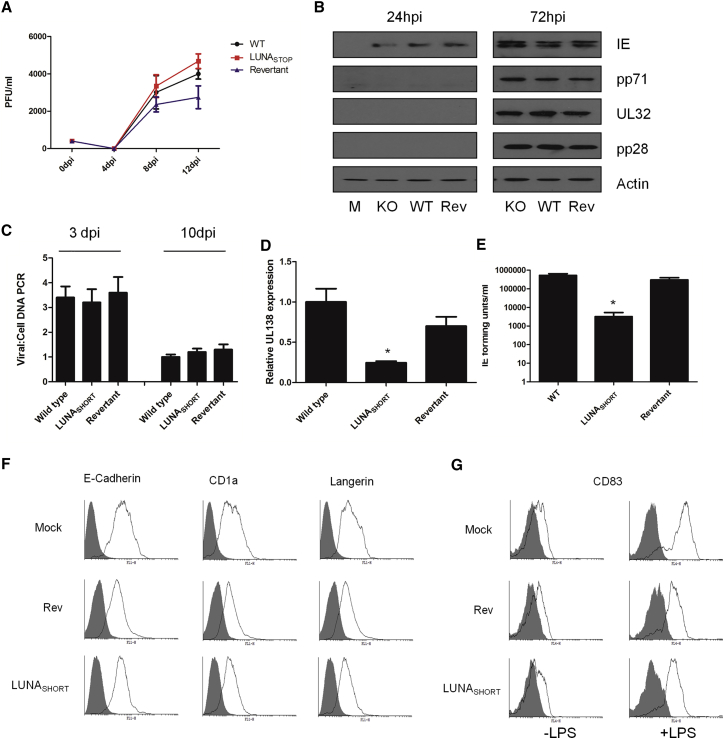
Figure 1.
LUNA Is Required for Efficient Reactivation in CD34+-Derived DCs
(A) HFFs were infected at an MOI of 0.1 with Merlin, LUNASHORT, or revertant virus, and the growth was measured over 14 days by titration of supernatants for infectious virus production every 2 days.
(B) Viral gene expression was also assessed by western blotting of mock infected cells (M) or cells infected with LUNASHORT (KO), wild-type (WT), or revertant (Rev) virus at 24 and 72 hrs post-infection (hpi).
(C) CD34+ cells infected with WT, revertant, or LUNASHORT were analyzed using qPCR for viral and cellular DNA levels at 3 dpi and then at 10 dpi after differentiation to an immature CD34+-derived DC phenotype.
(D) RNA isolated from WT revertant or LUNASHORT infected CD34+ cells at 7 dpi were analyzed for UL138 gene expression using qRT-PCR. All samples were normalized to GAPDH and then expressed relative to WT virus.
(E) CD34+ cells infected for 3 days to establish latency with WT, revertant, and LUNASHORT viruses were differentiated and matured into CD34+-derived DCs to induce reactivation and then co-cultured with fibroblasts. At 15 days, co-cultures were harvested and assayed for infectious virus production (IE forming units) on fresh fibroblasts. In (A) and (C)–(E), data are mean ± SD and represent triplicate analyses performed in two independent experiments. ∗p < 0.05.
(F) Cell surface phenotyping for E-cadherin, CD1a, and CD207/Langerin expression was performed on DCs derived from CD34+ cells latently infected with mock, revertant, or LUNASHORT virus. Isotype (shaded) and specific antibody (open) staining are shown.
(G) Cell surface phenotyping for CD83 expression was performed on immature (−LPS) and mature (+LPS) DCs derived from CD34+ cells latently infected with mock, revertant, or LUNASHORT virus. Isotype (shaded) and specific antibody (open) staining are shown.