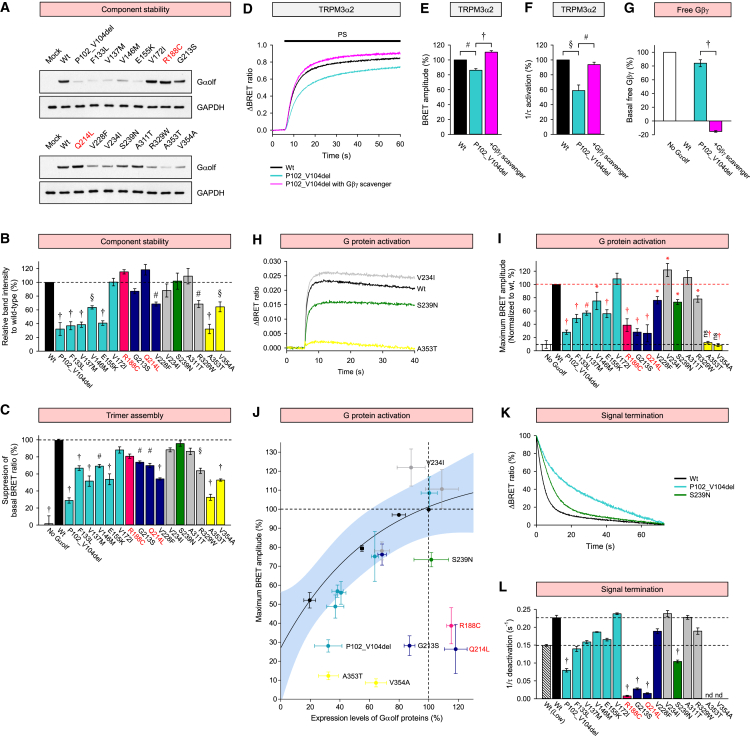
Figure 4.
Effects of the Mutations on the Expression Levels of Gαolf Proteins, Trimer Assembly of Gαolf/Gβ2γ7, on Coupling to GPCR and Signal Termination
(A) Western blotting analysis of Gαolf expression.
(B) Quantification of western blotting data in (A).
(C) Effect of mutations on trimer assembly measured by BRET. The ratio obtained without Gαolf is designated as 0% suppression.
(D–F) Effects of P102_V104del mutation on signaling to TRPM3α2 measured by Ca2+ influx with the CalFluxVTN BRET-based sensor. Increased Gβγ availability inhibits Ca2+ influx through the TRPM3α2 channel.
(D) Time course of PS-induced calcium influx through TRPM3α2.
(E and F) Quantification of the response amplitude (E) and activation rates (F) relative to wild-type (WT) Gαolf.
(G) Quantification of the relative amount of free Gβγ dimer.
(H–J) Effect of mutations on agonist-induced G protein activation measured with masGRK3ct BRET-based sensor.
(H) Time course of agonist-induced G protein activation.
(I) Quantification of the maximal response amplitude. The response amplitude obtained from WT Gαolf is designated as 100%.
(J) Correlation analysis of agonist-induced G protein activation versus expression level quantified from western blotting experiments.
(K and L) Effect of mutations on signal termination measured as quenching BRET signal in a masGRK3ct-based system upon the addition of an antagonist.
(K) Time course of signal termination upon antagonist addition.
(L) Quantification of the signal termination by single exponential analysis of the time course shown in (K). Wild-type Gαolf was transfected with a low (dashed) or standard amount (black) of Gαolf to estimate fluctuation caused by variation in expression.
Data are represented as mean ± SEM. See also Figure S3.